Often presents with acute hypertension and progressive renal failure and proteinuria as well as microscopic hematuria
![]() Possible to be normotensive but actually is hypertensive compared with baseline
Possible to be normotensive but actually is hypertensive compared with baseline
![]() Important to monitor baseline blood pressures
Important to monitor baseline blood pressures
History
Most patients present with Raynaud phenomenon and edema of fingers; arthralgias may be present.4 Rarely, esophageal symptoms occur first.
Physical Examination
1. Early in disease—edema of hands and feet
2. Later in disease course—thickening of skin of fingers (sclerodactyly)
3. Digital pits secondary to Raynaud phenomenon and other findings as outlined in CREST syndrome above
Laboratory Studies
• Greater than 90% of patients have positive ANA; nucleolar pattern associated with dSSc and centromere pattern with lSSc. ESR is often normal.6
• Anticentromere antibody testing is positive in 60% to 80% with limited disease (good prognosis if positive).6
• Antitopoisomerase-1 antibody (anti-Scl-70) can be positive in 20% to 40% with diffuse disease.
• Screen for visceral involvement with CBC, urinalysis, creatinine, pulmonary function tests, and diffusion capacity of carbon monoxide.
Imaging
Perform a chest radiograph to evaluate for pulmonary fibrosis; and perform esophageal motility studies.
Surgical Diagnostic Procedures
Skin biopsy.
Differential Diagnosis
• Limited diseases: mycosis fungoides, amyloidosis, porphyria cutanea tarda, reflex sympathetic dystrophy
• Diffuse diseases: idiopathic pulmonary fibrosis, primary biliary cirrhosis, GI dysmotility problems, SLE and overlapping syndromes
Treatment
Behavioral
Because of the sometimes disfiguring nature of the disease, counseling is recommended for supportive care. Protect extremities from cold temperatures.
Medications
• Raynaud disease should be managed with calcium-channel blockers (long-acting) or peripheral α-blockers. May also try pentoxifylline and stellate ganglion block.6
• Penicillamine for skin changes, in addition to moisturizing agents.
• Proton pump inhibitors and H2-receptor blockers for esophageal reflux symptoms. Promotility agents may be helpful.
• Monitor blood pressure and make early use of angiotensin-converting enzyme inhibitors.
Surgery
Progression of disease may eventually require lung and renal transplantation.
Referrals
Physical and occupational therapy should be involved early to prevent and treat functional loss secondary to skin changes. Rheumatology can help with confirmation of initial diagnosis and comanagement. Pulmonary and renal consults as needed with disease progression.
REFERENCES
1. Wasserman AM. Diagnosis and management of rheumatoid arthritis. Am Fam Physician 2011;84(11):1245–1252.
2. Beukelman T, Patkar NM, Saag KG, et al. 2011 American college of rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res 2011;63(4):465–482.
3. Hashkes PJ, Laxer RM. Medical treatment of juvenile idiopathic arthritis. JAMA 2005:294(13):1671–1684.
4. Gill JM, Quisel AM, Rocca PV, et al. Diagnosis of systemic lupus erythematosus. Am Fam Physician 2003;68(11):2179–2187.
5. Rahman A, Isenberg D. Systemic lupus erythematosus. N Engl J Med 2008;358:929–939.
6. Hinchcliffe M, Varga J. Systemic sclerosis/scleroderma: a treatable multisystem disease. Am Fam Physician 2008;78(8):961–969.
|
Fibromyalgia is a nonarticular rheumatic pain syndrome characterized by widespread musculoskeletal pain and tenderness on palpation at characteristic sites, called tender points. It is considered to be a disorder of pain regulation and classified as a central sensitization or pain amplification syndrome.1 Non-musculoskeletal symptoms often accompany the nonarticular musculoskeletal pain. Fatigue, sleep disturbance, anxiety or depression, headache, irritable bowel syndrome, dysmenorrhea, and paresthesias are among the more common non-musculoskeletal features. In 2010, preliminary criteria were recommended to capture these non-musculoskeletal symptoms, add additional regions of pain other than the tender points described in 1990, and suggest a symptom severity scoring system.2
DIAGNOSIS
History
Fibromyalgia occurs predominantly in Caucasian women (80% to 95%), who are 40 to 50 years old. The prevalence in the general population is 4% to 10%. Pain is the cardinal symptom and is widespread. According to the American College of Rheumatology, the pain must be above and below the waist, on both sides of the body, and along the axial skeleton.2 Non-musculoskeletal symptoms, especially anxiety and depression, are commonly found, and frequently overlap with chronic fatigue syndrome.
Physical Examination
According to the American College of Rheumatology Fibromyalgia Classification criteria, there must be tenderness on digital palpation (using 4 kg of force, or enough to blanch the nail bed of the thumb) in at least 11 of the following 18 (nine pairs) tender point sites:
• Occipital: bilaterally at the suboccipital muscle insertions a few centimeters below the nuchal ridge.
• Low cervical: bilaterally at the anterior aspects of the intertransverse spaces at C5–C7.
• Supraspinatus: bilaterally above the medial border of the scapular spine.
• Second rib: bilaterally at the second costochondral junction.
• Lateral epicondyle: bilaterally 2 cm distal to the epicondyle.
• Gluteal: bilaterally in the upper outer quadrants of the buttocks in the anterior fold of gluteus minimus.
• Greater trochanter: bilaterally just posterior to the trochanteric prominence.
• Knee: bilaterally at the medial fat pad proximal to the joint line.
Laboratory Studies
There are no routine laboratory markers for fibromyalgia. Specifically, the complete blood count and erythrocyte sedimentation rate are usually normal. Rheumatologic serologies are not diagnostic. Although abnormalities in T-cell subsets have been described, these tests are not currently recommended for routine use.3
TREATMENT
Injection
The tendinitis, bursitis, and costochondral tender points (lateral epicondyle, trapezius, greater trochanter, knee, and second rib) may be injected with lidocaine (Xylocaine) and/or corticosteroid.
Stretch and Spray
The myofascial trigger points of Travell (occiput, low cervical, supraspinatus, and gluteal tender points) may be stretched and have a vapo-coolant applied; alternatively, these may be injected with 0.5% procaine.3
Pharmacotherapy
Low-dose tricyclic antidepressants, such as amitriptyline (Elavil), 10 to 50 mg per day, or cyclobenzaprine (Flexeril) 10 to 30 mg per day, are recommended. Likewise, selective serotonin reuptake inhibitors, such as fluoxetine, 10 to 20 mg per day or paroxetine (Paxil) 5 to 10 mg per day, or low-dose dual (serotonin and norepinephrine) reuptake inhibitors such as duloxetine (Cymbalta) 60 mg per day, or venlafaxine (Effexor) 75 to 150 mg per day may be tried. The anticonvulsant pregabalin (Lyrica) was the first drug to attain FDA approval for the treatment of fibromyalgia and the dual reuptake inhibitor milnacipran (Savella) was the second to achieve approval.4 Nonsteroidal anti-inflammatory drugs and tramadol (Ultram) may also prove useful for the treatment of myofascial pain.4
Non-Pharmacologic Therapy
Exercise can be of significant benefit; low-impact aerobic activities such as walking, biking, swimming, or water aerobics are especially helpful.5 A 2008 systematic review of 34 studies of aerobic exercise and fibromyalgia found beneficial effects on aerobic performance, pain amelioration, and global well-being.6 Physical therapists and exercise physiologists familiar with fibromyalgia can provide helpful assistance and monitoring of progress. “Mind-body” interventions such as tai chi and yoga have demonstrated benefit in global well-being in a number of studies.6 Electromyographic biofeedback training and cognitive behavioral therapy also have proven benefit in patients with chronic fibromyalgia symptomatology.5–7
REFERENCES
1. Sarzi-Puttini P, Atzeni F, Mease PJ. Chronic widespread pain: from peripheral to central evolution. Best Pract Res Clin Rheumatol 2011;25(2):133–139.
2. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 2010;62(5):600–610.
3. Hara J. Myofascial syndromes (fibromyalgia and myofascial trigger points). In: Rakel R, ed. Saunders manual of medical practice. Philadelphia, PA: WB Saunders; 2006.
4. Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA 2004;292(19):2388–2395.
5. Busch AJ, Schachter CI, Overend TJ, et al. Exercise for fibromyalgia: a systematic review. J Rheumatol 2008;35(6):1130–1144.
6. Hadhazy VA, Ezzo J, Creamer P, et al. Mind-body therapies for the treatment of fibromyalgia. A systematic review. J Rheumatol 2000;27(12):2911–2918.
7. Miller FL, O’Connor DP, Herring MP, et al. Exercise dose, exercise adherence, and associated health outcomes in the TIGER Study. Med Sci Sports Exerc 2014;46(1):69–75.
|
GENERAL PRINCIPLES
Definition
Gout is an inflammatory disease caused by the deposition of uric acid crystals in and around joints, subcutaneous tissues (tophi), and kidneys.
Epidemiology
Gout primarily affects middle-aged men (ages 40 to 60) and postmenopausal women. The prevalence of gout has been increasing over the last two to three decades with self-reported prevalence of 3.9% of adults in the United States.1 Increasing rates of obesity, hypertension, type 2 diabetes mellitus, and chronic kidney disease may be contributing to the increase.2 Acute attacks of gout are less common in elderly people, in whom it may present insidiously or develop into chronic polyarticular arthritis.
Pathophysiology
Painful gouty attacks occur when uric acid crystals, a product of purine oxidation, are deposited in joints and subcutaneous tissues. Hyperuricemia is a marker for gout, but each can exist without the other. Defined as uric acid levels greater than 6.8 mg per dL,3 hyperuricemia is present in about 5% of the U.S. male population, of whom only a small minority will develop gout. Approximately 40% of patients have normal uric acid levels during an acute episode of gout;4 however, the risk of gout is proportional to the degree and duration of hyperuricemia. Levels normally rise during puberty in men and in postmenopausal women. It normally takes 20 to 30 years of hyperuricemia before a patient has a first episode of gouty arthritis. Primary hyperuricemia results from inborn errors of metabolism, either reduced excretion (90% of patients) or increased production (10%) of uric acid. Secondary hyperuricemia associated with gout can result from the use of thiazide diuretics, a high-purine diet (red meat and seafood), increased alcohol consumption, and obesity.5,6
Etiology
Risk of gout increases with increasing body mass index (BMI) and with weight gain over time. Weight loss, on the other hand, is protective against gouty attacks. Presence of hypertension is strongly associated with gout independent of renal failure and diuretic use.5 High levels of purine-rich seafood and red meat consumption may increase uric acid levels, whereas consumption of dairy products is protective. Increased consumption of purine-rich foods has no effect on clinical gout.6 Consumption of alcohol, in particular beer and spirits, increases the risk of gout. Ethanol metabolism increases serum lactate, which blocks renal uric acid excretion, leading to gouty attacks. Other factors provoking gouty attacks include rapid changes (either up or down) in serum uric acid levels, infection, surgery, renal failure, diuretic use, and emotional stress.
DIAGNOSIS
Clinical Presentation
Acute gout typically presents as an acutely painful monoarticular arthritis that may progress to chronic arthritis after years of progressively more severe and frequent episodes interspersed with variable symptom-free periods. The first metatarsophalangeal (MTP) joint is involved in 50% of initial acute gouty attacks (podagra), and 75% to 90% of patients with gout have first MTP involvement eventually. This is probably due to the microtrauma propensity of the first MTP and its relative coolness compared with the rest of the body. Gout severity ranges from vague aches and pains of low-grade polyarticular gout to dramatic attacks of extreme monoarticular pain to chronic polyarticular arthritis. Even in untreated gout, acute attacks resolve within several days to weeks. Presumptive diagnosis of acute gout can be made based on clinical signs and symptoms and a significant response to colchicine or nonsteroidal anti-inflammatory drugs (NSAIDs).
Timing of gouty attacks is quite variable and unpredictable, with the second and subsequent gouty attacks occurring weeks or decades later. However, gout recurs within 1 year in more than half of patients. As time passes, gouty attacks tend to occur more frequently, with greater severity, and with polyarticular involvement that is often refractory or poorly responsive to therapy. For unknown reasons, gouty attacks may be slightly more common in spring.
Gout in elderly people is often polyarticular and involves upper extremity joints (especially proximal interphalangeal joints and distal interphalangeal joints) and is associated with subcutaneous tophaceous deposits in the fingers, toes, and elbows. This may be misdiagnosed as rheumatoid arthritis.7
Women present 70% of the time with polyarticular disease rather than the classic monoarticular arthritis seen in men.8
Physical Examination
During acute gouty attacks, pain, swelling, redness, and exquisite tenderness develop suddenly in the joint and surrounding area, peaking within 24 to 48 hours. The heel, ankle, knee, midtarsal joints, and olecranon bursa can all be initially involved but less frequently than the first MTP. Acute polyarticular gout is less common but has a more dramatic presentation. Acute polyarticular gout can cause a high fever and leukocytosis, making it difficult to distinguish from septic arthritis.
Frequent, recurrent acute gouty attacks can lead to chronic gout, in which, joint swelling, deformity, and disability may be present. Deformity is caused by tophi, deposits of monosodium urate crystals in the soft tissue overlying joints.
Laboratory Studies
The presence of monosodium urate crystals in synovial fluid or tophi is diagnostic of gout. Negative joint cultures and hyperuricemia contribute to the presumptive diagnosis.
Pathologic Findings
In acute gout, needle-shaped urate crystals are found inside synovial fluid phagocytes or free within tophaceous deposits. These are strongly negatively birefringent under a polarized microscope lens. The calcium pyrophosphate crystals of pseudogout are, on the other hand, weakly positively birefringent and rhomboid-shaped.
Imaging
There are no imaging studies that are diagnostic of acute gouty arthritis. The classic radiographic finding of chronic gout is sharply marginated erosions proximal to the joint space with an overlying rim of cortical bone. Uric acid calculi can be seen as filling defects on intravenous pyelograms.
Classification
• Acute gout describes acute painful attacks of arthritis induced by urate crystal deposition. During the intervals between acute gouty attacks, patients with early gout are virtually asymptomatic.
• Intercritical gout describes these asymptomatic periods. Urate crystals can be aspirated from quiescent joints during these interval periods; therefore, the finding of urate crystals during an acute episode provides little reassurance of a nonseptic cause; antibiotic therapy should be based on clinical presentation, Gram stain, and culture.9 Crystals remain present in joints as long as hyperuricemia persists; when serum uric acid levels are reduced to normal, urate crystals slowly dissolve and finally disappear from the joint.
• Chronic tophaceous gout has become increasingly rare due to more widespread drug treatment of hyperuricemia and gout. Tophi without prior episodes of gouty arthritis are unusual because they normally occur after gout has been present for more than 10 years. Tophi can occur anywhere but tend to occur in the helix of the ear, proximal ulnar surface of the forearm, olecranon, Achilles tendon, prepatellar bursa, or near active joints. Secondary gout is caused by overproduction or underexcretion of uric acid due to drugs or other disease processes. Overproduction of uric acid occurs in myeloproliferative and lymphoproliferative disorders, polycythemia, hemolytic anemia, multiple myeloma, and other malignancies. Renal disease, diuretics, low doses of salicylates, chronic lead intoxication (“saturnine gout”), nicotinic acid, alcohol, ethambutol, and pyrazinamide all cause underexcretion of uric acid. Acute uric acid nephropathy occurs primarily in patients undergoing chemotherapy for hematologic or myeloproliferative disorders and can be prevented by several days of allopurinol administration and adequate hydration before initiation of chemotherapy.
Differential Diagnosis
Gout can be misdiagnosed as inflammatory osteoarthritis, particularly given that erosions on radiographs are seen in both conditions. Gout may be mistaken for rheumatoid arthritis because tophi may resemble rheumatoid nodules, and rheumatoid factor often becomes weakly positive as people age. It may be difficult to differentiate cellulitis or septic arthritis from gout, particularly when a low-grade fever, leukocytosis, redness, or desquamation is present. The term pseudogout, for calcium pyrophosphate deposition disease, belies the difficulty in clinically differentiating it from gout. For definitive diagnosis, joint fluid must be aspirated for culture and a search for urate crystals.
Treatment Approach
All patients with an established gout diagnosis should receive patient education on the role of uric acid, risk factors, and the natural course of the disease. Counseling should include diet and lifestyle recommendations. A comorbidity checklist should be used to consider secondary causes of hyperuricemia, and nonessential medications, which induce hyperuricemia, should be considered for elimination.2 Acute gouty arthritis attacks should be treated with pharmacologic therapy, preferably initiated within 24 to 48 hours of symptom onset. The choice of pharmacologic agent should depend largely on the severity of pain and the number of joints involved, acknowledging that combination therapy may be an appropriate option if pain is severe or one to two large joints are involved.10
Medications
NSAIDs, colchicine, and systemic corticosteroids are all considered appropriate first-line options for an acute gouty attack. Specific medication choice should be based upon patient preference, prior response to treatment, and associated comorbidities.10
• NSAIDs are recommended for use in acute gout and three (indomethacin, naproxen, and sulindac) have specific FDA approval for gout treatment. There is no evidence that one specific NSAID is superior to the others, but all NSAIDs should be prescribed at anti-inflammatory dosing levels and continued at that level until symptoms are completely resolved. Dosing may be tapered in patients with renal failure. COX-2 inhibitors are an option in patients with gastrointestinal (GI) contraindications or NSAID intolerance; celecoxib 800 mg one time followed by 400 mg on day 1 then 400 mg bid for 1 week was shown to be equal to indomethacin in acute gout.10 NSAIDs can cause GI toxicity (nausea, abdominal discomfort, GI bleeding, peptic ulcer disease), nephrotoxicity, and central nervous system side effects (headache, dizziness, confusion). Therefore, they must be used with caution, especially in the elderly or in patients with underlying disease. Colchicine terminates most acute gouty attacks within 6 to 12 hours; however, it is limited by its GI side effects and is often poorly tolerated by the elderly. Colchicine is much more effective if given within the first 12 to 24 hours of an acute attack. Its mechanism of action is not entirely known but apparently reduces the inflammatory response to urate crystals and diminishes phagocytosis. Recommended dosing has changed, with the most recent guidelines now suggesting a loading dose of 1.2 mg of colchicine followed by 0.6 mg orally 1 hour later, and then 0.6 mg up to three times daily thereafter.10 Possible bone marrow toxicity limits the total dose for a single day to 4.8 mg (less if the patient has hepatic or renal disease). Colchicine should be used prophylactically when initiating uric acid–lowering therapy to prevent precipitating an acute gouty attack. The optimal duration of prophylaxis is unknown, but it can usually be stopped after the uric acid level is brought down to a normal range for 2 months. Colchicine, 0.6 mg bid, is usually started several days before the urate-lowering agent is started. Corticosteroids are an additional first-line treatment option. Prednisone or prednisolone at 5 to 10 mg per kg per day for 5 to 10 days can be given followed by discontinuation, or 2 to 5 days at the full dose may be followed by a 7- to 10-day taper. Rebound can be avoided by using colchicine prophylactically, 0.6 mg PO bid, which should then be discontinued 6 to 8 weeks later. In patients with gout involving one or two large joints (ankle, knee, wrist, elbow, hip, or shoulder) or who are unable to tolerate oral therapy, intra-articular corticosteroid injections are useful. These usually result in resolution of an acute gouty episode within 12 to 24 hours.
• Combination therapy may be appropriate for patients with severe acute gout (pain >7 on a 10-point pain scale) and for patients with an acute polyarthritis or involvement of more than one large joint, full doses of two of the pharmacologic modalities recommended above may be used in combination. The exception to this is a combination of corticosteroids and NSAIDs, which would pose an unacceptably high risk of GI side effects.10
• Allopurinol (Zyloprim) is a xanthine oxidase inhibitor that decreases the production of uric acid. For patients with recurrent gouty attacks (more than one per year), renal stones, renal damage, or uric acid–lowering therapy should be initiated. Allopurinol is effective in most patients regardless of the source of hyperuricemia (overproduction or underexcretion) because it produces a more soluble metabolite. A 24-hour urinary uric acid determination to differentiate urate overproduction from underexcretion is therefore unnecessary in most patients. Allopurinol is also better tolerated than uricosuric agents, has fewer drug–drug interactions, is effective in patients with renal failure or nephrolithiasis, and is used in a single daily dose. In patients receiving chemotherapy, allopurinol should be used when daily uric acid excretion exceeds 800 mg per 24 hours in male patients and 750 mg per 24 hours in female patients. Allopurinol may be started at 100 mg daily with food and increased at weekly intervals by 100 mg until a serum uric acid level of 6 mg per dL or less is attained. The average effective dose for mild gout is 200 to 300 mg per day, although some patients need 400 to 600 mg per day, particularly those with tophaceous gout or those on cancer chemotherapy. Enough fluids should be taken to keep daily urine output greater than 2 L. If an acute attack occurs while taking allopurinol, the dose should be maintained as is and the attack treated as usual (e.g., NSAIDs, colchicine). In elderly patients, a starting dose of 50 to 100 mg on alternate days, to a maximum daily dose of 100 to 300 mg based on the patient’s creatinine clearance and serum urate level, decreases the risk of hypersensitivity reactions.8 Life-threatening hypersensitivity reactions to allopurinol involving skin, kidney, and liver occur rarely but are being recognized with increasing frequency. The most frequent adverse reactions to allopurinol are skin rash, GI reactions (diarrhea, nausea, and alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase elevations), and acute attacks of gout, which can be minimized by proper use. Renal function must be monitored in patients taking thiazide diuretics. Allopurinol may also cause a rash in patients taking ampicillin or amoxicillin, and it may potentiate anticoagulants.8
• Febuxostat (Adenuric), a newer xanthine oxidase inhibitor, is also considered a first-line urate-lowering therapy, with no evidence of clear superiority between febuxostat and allopurinol. Febuxostat has been shown to be effective at reducing urate levels to <6 mg per dL. Some evidence indicates that it is slightly more effective at reducing uric acid levels, but with a slightly higher risk of precipitating an acute gout attack. The increased rate of acute attacks does not persist over time, and can be managed by prophylaxis with anti-inflammatory agents or colchicine.11
• Uricosuric drugs are indicated for patients who need urate-lowering therapy but are intolerant or unresponsive to xanthine oxidase inhibitors. These medications block renal tubular reabsorption of uric acid. As with allopurinol, they should never be started during an acute attack but should be maintained if started previously. Before using these agents, a 24-hour urine for creatinine clearance and urine uric acid should be performed, as uricosuric drugs are ineffective for a glomerular filtration rate less than 50 mL per minute and can increase the risk of urate stones if the urinary uric acid is already elevated (>800 mg per 24 hours). Urate stone formation can be minimized if patients maintain a high fluid intake and alkalinize the urine. These medications include probenecid and sulfinpyrazone.
• Probenecid (Benemid) is started at 250 mg bid for 1 week and then 500 mg bid after that. The dose is increased by 500 mg every 1 to 2 weeks until the serum urate level is normal or the 24-hour uric acid excretion is at or below 800 mg. The usual effective dose is 500 mg twice daily. Probenecid is well tolerated but is not effective in even mild renal insufficiency, and should not be used in patients with creatinine >2. It also should not be used with salicylates, as they antagonize its action. It can also raise plasma levels of penicillin, sulfonylureas, and NSAIDs. In patients with glucose-6-phosphate dehydrogenase deficiency, it can cause hemolytic anemia.
Special Therapy
Arthroscopic Removal of Urate Crystals
A small cohort study demonstrated that surgical removal of urate crystals from the first MTP joint reduced gout flares and improved function compared with standard therapy in patients followed for 4 years.12
Counseling
Although no randomized controlled trials have proved the effectiveness of counseling patients on decreasing the frequency of gouty attacks, it is prudent for the physician to counsel patients with a gout history about factors known to worsen the course of the disease. Counseling should suggest dietary decrease in meat and seafood, and a corresponding increase in dairy product consumption. Advice on weight loss may also be beneficial. The American College of Rheumatology advises a thorough patient education effort that includes a basic explanation of the disease, treatment options, and objectives of treatment, including the reduction of uric acid.2
Risk Management
Although there are multiple disease states that increase risk of gouty attacks, the presence of gout generally does not increase risks for other diseases or have multiple complications. One exception to this is in the kidney, namely, interstitial renal disease and nephrolithiasis. Men with gout have a twofold increased risk for developing kidney stones compared with men without gout. Therefore, it may be prudent to counsel men to increase fluid intake and decrease salt consumption to modify this risk factor.13
REFERENCES
1. Zhu YM, Pandya BJ, Choi HK. Prevalence of goat and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011;63(10):3136–3141.
2. Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American college of Rheumatology guidelines for management of gout. Part I: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res 2012;64(10):1431–1446.
3. Neogi T. Clinical practice. Gout. N Engl J Med 2011;364(5):443–452.
4. Schlesinger N, Baker DG, Schumacher HR. Serum urate during bouts of acute gouty arthritis. J Rheumatol 1997;24(11):2265–2266.
5. Choi HK, Atkinson K, Karlson E, et al. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005;165(7):742–748.
6. Choi HK, Atkinson K, Karlson E, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350(11):1093–1103.
7. Sturrock RD. Gout: easy to misdiagnose. BMJ 2000;320(7228):132–133.
8. Lally EV, Ho G, Kaplan SR. The clinical spectrum of gouty arthritis in women. Arch Intern Med 1986;146(11):2221–2225.
9. Johnson JR. Diagnosis of intercritical gout. Ann Intern Med 2000;132(10):843.
10. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American college of Rheumatology guidelines for management of gout. Part II: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res 2012;64(10):1447–1461.
11. Tayar JH, Lopez-Olivo MA, Suarez-Almazor ME. Febuxostat for treating chronic gout. Cochrane Database Syst Rev 2012;11:CD008653. doi: 10.1002/14651858.CD008653.pub2
12. Wang CC, Lien SB, Huang GS, et al. Arthroscopic elimination of monosodium urate deposition of the first metatarsophalangeal joint reduces the recurrence of gout. Arthroscopy 2009;25(2):153–158.
13. Kramer HJ, Choi HK, Atkinson K, et al. The association between gout and nephrolithiasis in men: the health professionals’ follow-up study. Kidney Int 2003;64(3):1022–1026.
|
GENERAL PRINCIPLES
Definition
Overuse injuries occur when forces applied over time to a bone, muscle, tendon, or ligament exceed the ability of those tissues to adapt to the forces. Repetitive microtrauma leads to local tissue damage in the form of cellular and extracellular degeneration, and most likely occurs more often with a sudden change in mode, intensity, or duration of activity.
Epidemiology
More than 50% of pediatric sports injuries are overuse injuries.1 More than 50% of occupational illnesses involve overuse from repetitive motion injuries.
Pathophysiology
The muscle-tendon unit is the most common site of injury, but other structures such as bone, cartilage, ligament, bursa, and fascia may be involved. In children, the growth plates are also vulnerable to the stress of repetitive injury.
Etiology
The etiology is multifactorial, involving a pathway of repetitive microtrauma and local tissue injury. Modification of both intrinsic and extrinsic risk factors is essential for injury treatment and prevention.
• Intrinsic risk factors are inherent characteristics of an individual’s body. These include muscle inflexibility, muscle weakness, joint laxity, previous injury, anatomic malalignment, and lower extremity asymmetry. Frequently a deficit in one body area may affect function in another region.
• Extrinsic risk factors include equipment malfunction, training errors, environmental conditions, biomechanical errors, and ergonomic problems.1
TREATMENT APPROACH
• Initial treatment aims to reduce the inflammatory process with relative rest and ice.
• Ice. Applied for 15 to 30 minutes every 2 to 6 hours over the first 24 to 48 hours as necessary.
• Nonsteroidal anti-inflammatory drugs (NSAIDs). Controversial in acute and chronic injury—may reduce inflammation, but may also slow inflammatory-mediated healing.
• Modalities such as ultrasound, electrical stimulation, or iontophoresis may reduce initial inflammation.
• Corticosteroid injection may reduce inflammation for certain injuries (e.g., subacromial bursitis and de Quervain tenosynovitis). Side effects may include subcutaneous fat atrophy or necrosis, depigmentation, hyperpigmentation, tendon rupture, accelerated joint destruction, or infection. Diagnostic information may be obtained if injection of local anesthetic (1% lidocaine or 0.5% bupivacaine) reduces local pain.
• Definitive treatment includes identification and modification of predisposing risk factors. Exercises, especially eccentric exercises, address deficits in strength, flexibility, and proprioception. Rehabilitation efforts include correcting biomechanical abnormalities, such as poor throwing motion, as well as addressing poor ergonomics, such as chair height or computer screen adaptation. Incomplete rehabilitation of any previous injury is also addressed.
ROTATOR CUFF TENDINOPATHY
Diagnosis
Clinical Presentation
Rotator cuff tendinopathy is a common cause of nontraumatic shoulder pain in both children and adults. Pain is frequently insidious, and there is often a nocturnal component. In adults, impingement of the supraspinatus tendon as it runs beneath the subacromial arch is the most common cause of tendinopathy.
Physical Examination
Pain with shoulder abduction is the most common finding. Provocative maneuvers with forward flexion and internal rotation (Neer or Hawkins signs), which force the humerus into the subacromial space, will often reproduce symptoms.
Imaging
Radiographs are helpful in excluding other causes of persistent (greater than 6 weeks) shoulder pain, including calcific tendonitis, glenohumeral arthrosis, and bone tumors. A supraspinatus outlet view is helpful to evaluate the bony morphology of the anterior acromion. For diagnostic uncertainty coupled with persistent pain or for treatment nonresponders, magnetic resonance imaging (MRI), potentially with gadolinium contrast, is useful to help guide treatment.
Treatment
Nonoperative
Initial treatment includes ice, a limited course of NSAIDs, and avoiding aggravating factors. A subacromial corticosteroid injection combined with local anesthetic may confirm symptom source and reduce pain. More definitive therapy involves maximizing glenohumeral motion, stabilizing the scapulothoracic articulation, strengthening the rotator cuff, and addressing biomechanical or ergonomic errors.2
Operative
For adults with persistent impingement symptoms, MRI completion and referral for surgery to relieve bony impingement may be necessary.
EPICONDYLITIS
Diagnosis
Clinical Presentation
Epicondylitis is the most common overuse problem in the elbow. Pain, often insidious, is present over the affected epicondylar region. Excessive wrist extension precedes lateral epicondylitis, whereas excessive wrist flexion precedes medial epicondylitis. Lateral epicondylitis is more common than medial epicondylitis.
Physical Examination
With lateral epicondylitis, “tennis elbow,” pain radiates distally along the extensor forearm muscles. Symptoms are reproduced with resisted wrist extension while the forearm is pronated and with resisted supination. With medial epicondylitis, “golfer’s elbow,” pain is commonly elicited with resistance to wrist flexion and forearm pronation. Reduced grip strength may be noted with either condition.
Imaging
For treatment nonresponders, MRI can help to identify tendon tears that may warrant surgical intervention.
Treatment
Nonoperative
Initial treatment includes ice with friction massage, relative rest, and a short course of NSAIDs. Avoiding pronation activities may be useful in lateral epicondylitis. A counterforce brace distal to the affected epicondyle may be useful, as may a short period of wrist splint immobilization to reduce the aggravating motion. When pain persists, a corticosteroid injection at the site of maximal tenderness may improve symptoms. Care should be exercised in a medial epicondyle injection to avoid infiltrating the ulnar nerve. Rehabilitation should include stretching and strengthening, especially eccentric strengthening of the forearm musculature, and biomechanical or ergonomic modifications.3
Operative
Rarely, treatment nonresponders may require surgical debridement for relief of symptoms.
CARPAL TUNNEL SYNDROME
Diagnosis
Clinical Presentation
Carpal tunnel syndrome is an entrapment neuropathy caused by compression of the median nerve between the transverse carpal ligament and the flexor tendons of the wrist. Symptoms of pain, paresthesias, and/or numbness occur in the sensory distribution of the median nerve, especially the index finger. Presentation occurs bilaterally in up to 50% of cases. Commonly affected groups include middle-aged women and workers with repetitive manual labor. Tasks requiring a strong grip with wrist flexion and extension or that have vibration exposure are at greatest risk. The majority of patients experience pain that is strong enough to awaken them from sleep, with relief obtained by shaking their wrist (“flick sign”). The syndrome affects up to 3% of the population, and is three times more common in women.4
Physical Examination
Pain in the distribution of the median nerve often can be elicited with percussion over the median nerve (Tinel sign), hyperflexion of the wrist (Phalen test), or compression of the median nerve (Durkan test). Severe cases often result in thenar atrophy. Diagnostic confirmation can be made with electromyography (EMG) and nerve conduction study (NCS) testing.
Imaging
Wrist radiographs are rarely of help, although a special carpal tunnel view can rule out other anomalies, such as a fracture of the hook of the hamate.
Treatment
Nonoperative
Initial identification of aggravating causes, including work modification, may be all that is needed for mild cases. Other early interventions may include nocturnal or job-specific splinting, while NSAID use is less certain to provide benefit.5 Direct injection of corticosteroid into the carpal tunnel often alleviates symptoms initially, but a majority still progress to surgery.5,6
Operative
For refractory cases, or when EMG/NCS reveals moderate to severe nerve compression, median nerve decompression by incision of the transverse carpal ligament is recommended.
DE QUERVAIN TENOSYNOVITIS
Diagnosis
Clinical Presentation
De Quervain disease is characterized by inflammation of the first dorsal compartment of the wrist (containing the extensor pollicis brevis and abductor pollicis longus) as it passes over the radial styloid. Excessive repetitive hand motion, especially involving radial and ulnar wrist deviation, is a frequent cause.
Physical Examination
Pain is exquisitely reproduced by sharp ulnar deviation of the hand with the thumb flexed (Finkelstein test).
Imaging
Imaging is rarely of help in making the diagnosis. Degenerative arthritis of the first carpal–metacarpal joint may be seen, but this problem manifests pain in a different location.
Treatment
Nonoperative
First-line treatment is local corticosteroid injection into the tendon sheath. Recent evidence suggests little to no added benefit from NSAIDs and/or thumb spica splinting versus injection alone. Iontophoresis may also be useful as an early modality.7
Operative
When nonoperative treatments fail to alleviate symptoms, surgical release of the compartment may be necessary.
TRIGGER FINGER
Diagnosis
Clinical Presentation
Trigger finger involves a stenosing tenosynovitis of the flexor tendons of the hand. Although the complaint may be of pain at the proximal interphalangeal (PIP) joint, the problem is actually located at the palmar surface of the metacarpophalangeal (MCP) joint.
Physical Examination
Most commonly, inflammation involves the A1 pulley, the first of five pulleys that guide the flexor tendon into the finger. Locking or triggering may occur as the stenosed tendon becomes trapped in the pulley.
Treatment
Nonoperative
Treatment is focused on a corticosteroid tendon sheath injection. For persistent or recurrent symptoms, a second injection several months later with application of a trigger-finger splint may be helpful, provided that the first injection yielded some symptomatic relief.
Operative
For patients who do not respond after a first injection, or whose symptoms recur after a second injection, surgical release of the pulley will be necessary.
PATELLOFEMORAL PAIN SYNDROME
Diagnosis
Clinical Presentation
Patellofemoral pain syndrome (PFPS) or patellofemoral dysfunction refers to anterior knee pain arising from the patellofemoral joint. Symptoms can range from mild activity-related knee pain to severe pain limiting ordinary activities. Pain typically worsens after prolonged sitting with the knees flexed (“theater sign”) or with activities that require repetitive knee flexion, such as stair climbing/descending or running.
Physical Examination
Palpation often elicits tenderness of the medial or lateral patellar facets. Pain increases with downward pressure applied to the superior pole of the patella during isometric quadriceps contraction (patellar grind/compression test). Provocative maneuvers of the ligaments or menisci will be negative.
Imaging
Radiographs are most helpful in excluding other diagnoses, such as osteochondritis dissecans. A patellar “sunrise” or “merchant” view, which can reveal patella tilting, most commonly to the lateral side, is suggestive but not diagnostic of PFPS.
Treatment
Nonoperative
Treatment should be aimed at reducing pain and eliminating predisposing risk factors. Activities with significant quadriceps loading, such as stair climbing, should be minimized. After a brief period of relative rest, ice, and NSAIDs, a rehabilitation program should focus on correcting lower extremity deficits in flexibility, strength, and proprioception. Abnormal foot biomechanics, such as excessive pronation, should be addressed. Patellar taping techniques or bracing to correct malalignment may reduce symptoms.8
Operative
Surgery is rarely needed for PFPS. In refractory conditions, release of the lateral retinaculum can be considered.
ILIOTIBIAL BAND SYNDROME
Diagnosis
Clinical Presentation
Iliotibial (IT) band syndrome refers to lateral hip or lateral knee pain due to chronic friction of the IT band over the greater tuberosity of the femur and/or the lateral femoral condyle. Pain, usually achy in quality, is worsened by activities with repetitive knee and hip flexion, such as distance running and cycling.
Physical Examination
Palpation often elicits tenderness of the inflamed IT band over the lateral femoral condyle or greater trochanter, particularly if an associated bursitis has developed. Tightness of the IT band is usually demonstrated through a positive Ober test.8
Imaging
Although IT band syndrome is usually a clinical diagnosis, occasionally an MRI is obtained to exclude other potential surgical findings that may present similarly with knee lateral joint line tenderness, such as lateral meniscus injuries.
Treatment
Nonoperative
Treatment should be aimed at reducing pain and eliminating predisposing risk factors. After a brief period of relative rest, ice, and NSAIDs, a rehabilitation program should focus on stretching the IT band and hamstrings and strengthening the hip abductors, along with correcting training errors, such as excessive downhill running. Friction massage, such as with foam rolling, may help to break up the areas of thickening and scarring. Localized corticosteroid injection may be offered when pain and swelling persist despite more conservative measures.
Operative
In cases that are unresponsive to nonoperative management, surgical release may be considered.
PREPATELLAR BURSITIS–“HOUSEMAID’S KNEE”
Diagnosis
Clinical Presentation
Chronic inflammation of the prepatellar bursa results from recurrent trauma, such as repetitive kneeling.
Physical Examination
Acute trauma may also lead to immediate swelling of the prepatellar bursa, as can underlying infection. If infection is suspected, aspiration for Gram stain and culture should be performed.
Treatment
Nonoperative
Protective padding is an essential part of treatment in recurrent cases. When infection is suspected, antibiotics should be instituted after cultures are obtained. Chronic inflammation may respond to a corticosteroid injection.
Operative
For refractory cases, surgical excision of the bursa may be necessary.
MEDIAL TIBIAL STRESS SYNDROME—“SHIN SPLINTS”
Diagnosis
Clinical Presentation
Both the name and exact cause of this entity, which is common among runners, dancers, and other athletes, continue to generate controversy. Pain typically increases at the onset of activity, improves with continued activity, and resolves with rest; however, symptoms often intensify with higher activity levels. Contributing intrinsic factors include anatomic malalignment and deficits in flexibility and strength of the lower extremity. Extrinsic factors include inadequate or worn-down footwear, insufficient warm-up, uneven or hard running surfaces, and rapid advancement of a training regimen.
Physical Examination
The diagnosis is based on diffuse pain and tenderness at the posteromedial aspect of the tibia.
Imaging
Radiographs are useful primarily to rule out other disorders, such as tibial or fibular stress fractures.
Treatment
Nonoperative
Initial treatment includes relative rest, ice massage, and NSAIDs. Occasionally symptoms can persist for weeks, especially if the athlete continues at high levels of activity. Total rest, including cessation of all athletic activities, may be necessary to resolve symptoms. Definitive treatment also includes identification and correction of modifiable risk factors, along with a gradual increase to premorbid activity levels.9
PLANTAR FASCIITIS/FASCIOSIS
Diagnosis
Clinical Presentation
Heel pain from plantar fasciitis is caused by degenerative thickening (and potentially inflammation) of the aponeurosis that arises from the calcaneus and inserts distally on the proximal phalanges. The pain is typically most intense upon arising in the morning, with symptom improvement during activity. Risk factors include obesity, excessive foot pronation, poor lower extremity flexibility, planus or cavus foot type, and calf weakness. Patients may be active or sedentary.
Physical Examination
Frequently, severe pain and tenderness localize to a single plantar area slightly anterior to the medial calcaneal tubercle.
Imaging
Radiographs are of little diagnostic help. Although 50% of patients with plantar fasciitis show heel spurs on the anterior calcaneus, this finding is also present in up to 25% of asymptomatic individuals.
Treatment
Nonoperative
Initial measures should include relative rest, appropriate shoe support including shoe inserts, and calf stretching. As a second step, custom-made night splints, physical therapy, and corticosteroid injection may be helpful. Extracorporeal shock wave therapy may be effective in select populations, such as runners with chronic heel pain.10
Operative
In rare cases, surgical intervention is needed to relieve symptoms.
ACHILLES TENDINOPATHY
Diagnosis
Clinical Presentation
Patients complain of pain and swelling in the Achilles tendon, usually 4 to 7 cm proximal to the calcaneal insertion. Activity will exacerbate the pain.
Physical Examination
Dorsiflexion of the foot or local palpation reproduces the symptoms, and the patient may have an antalgic gait.
Treatment
Nonoperative
Ice, NSAIDs, relative rest, and gentle calf stretching should be the first treatments. Early referral for physical therapy should be added to address flexibility, resolve strength deficits (eccentric strengthening exercises are key), and correct biomechanical abnormalities. Recently, topical nitroglycerine has been demonstrated as a useful adjunct in promoting healing.3
STRESS FRACTURES
Diagnosis
Clinical Presentation
When repetitive weight-bearing activity causes the bony architecture to exceed a given threshold, a stress injury can occur. The process begins as a stress reaction; however, if allowed to progress unabated, a stress fracture will result. Risk factors include sudden increases in training regimen or activity level, osteopenia, poor biomechanics, and hormonal factors. In general, women are more susceptible than men.
History
Symptoms begin with progressive localized pain with activity that resolves with rest. If untreated, pain will occur with lower levels of activity, and eventually with rest. Approximately 90% to 95% of stress fractures involve the lower extremity, although specific sports can be associated with upper body injuries (rowing: rib fractures; throwing: humerus/ulnar fractures).
Physical Examination
Palpation usually elicits point tenderness over the bone with distal lower extremity involvement. In proximal lower extremity fractures, the pain may be more ill-defined. The most common sites for injury are the tibia, metatarsals, and fibula.
Imaging
Radiographs are often normal until 2 to 4 weeks from symptom onset, when reactive sclerosis of the bone appears. Bone scans may provide diagnostic confirmation as early as 72 hours from symptom onset, but early findings may be inconclusive. MRI is an expensive but very accurate diagnostic tool, with detection as early as 24 to 48 hours from symptom onset.
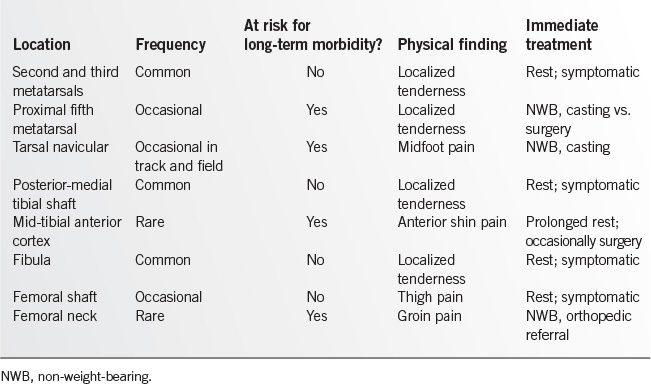
Classification
Stress fractures can be divided into high-risk and low-risk fractures based on their potential for long-term morbidity (Table 15.5-1). High-risk fractures have greater potential for complications such as delayed union, nonunion, bony displacement, or completed fracture. Orthopedic consultation is advisable for these injuries, which require individualized and prolonged management.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree