and Alena Skalova2
(1)
Departamento de Ciências Biomédicas e Medicina, Universidade do Algarve, Faro, Portugal
(2)
Department of Pathology, Medical Faculty Charles University, Plzen, Czech Republic
7.1 General Aspects on Salivary Gland Cancer Incidence
Head and neck cancer is the sixth most common form of cancer worldwide, and salivary gland malignancies constitute 3–5 % of all head and neck cancers. Malignant salivary gland tumours are still rare with an annual incidence of 2.5–3.0/100.000 and represent only 0.5 % of all malignancies. It is important to recall that the relative frequency of individual salivary gland tumours is extremely difficult to estimate with accuracy. This is due to the fact that available data derive from numerous different settings, some studies include minor salivary gland tumours only, many series are relatively small, and there are also real differences between different parts of the world. For example, an especially high incidence of pleomorphic adenoma has been reported from South Africa. The Japanese population appears to have a higher incidence of intraoral benign tumours, more marked tendency for female predominance, higher incidence of palatal involvement, etc. [7, 56, 128, 131, 158, 172, 181]. Geographical and racial differences play an important role in the relative frequency of particularly mucoepidermoid and adenoid cystic carcinoma. For example, a Finnish study reports adenoid cystic carcinoma to be the most common malignant tumour followed mucoepidermoid (27 and 19 %, respectively) [101]. Also a study from India showed adenoid cystic carcinoma to be more common than mucoepidermoid carcinoma (25 and 18 %, respectively) [166], as did a recent Danish study. Bjørndal and associates reviewed 952 salivary gland malignancies from three nationwide registries from 1990 to 2005. The study also incorporated a histological revision according the 2005 WHO classification, and they found adenoid cystic carcinoma being the most common malignancy, followed by mucoepidermoid carcinoma, 25.2 and 16.9 %, respectively [22]. The result of a large study from the UK, however, supported the more prevailing opinion that mucoepidermoid carcinoma is more common than adenoid cystic carcinoma (33 and 24 %, respectively) [85]. Further, a Chinese study of a large series of 737 intraoral salivary gland tumours suggests several differences to the western world. It appears that in southwest China, there is a higher incidence of intraoral minor salivary gland tumours and there is a higher incidence of malignant than benign tumours compared to other countries. There is also a higher incidence of myoepithelioma and an apparent absence or paucity of canalicular adenoma in the Chinese population [182]. The incidence of, for example, intraoral mucoepidermoid carcinoma varies a lot between different countries in the world. Studies from, for example, Japan and Uganda give a relative frequency of only around 10 % of all salivary gland tumours, benign and malignant, but as much as 25.3 % in Libya. Studies from the USA show that mucoepidermoid carcinomas represent approximately 20 % of intraoral tumours whilst in South American countries like Venezuela and Brazil the figures are 29.0 and 38.8 %, respectively [27].
Hence, all incidence data given below, and in other parts of this book as well, shall be interpreted with great caution and are meant as guidelines only. The risk of a salivary tumour to be malignant is smallest in the parotid gland where approximately 15 % of neoplasms are malignant, whilst 30–40 % of submandibular tumours are malignant. In minor salivary glands, 45–50 % of tumours are malignant, but of sublingual, tongue and lacrimal tumours, as much as 90 % are malignant. The most common site for intraoral salivary tumours is the palate, followed by the upper lip and the buccal mucosa, and these three sites account for approximately 75 % of all intraoral salivary gland tumours. A very crude estimate of all malignancies in major and minor salivary glands together, mucoepidermoid carcinoma (MEC) is the most common malignant tumour representing 30–35 % of salivary malignancies and 10 % of all salivary gland tumours, benign and malignant. Adenoid cystic carcinoma (ADCC) represents approximately 20 % of malignant salivary gland tumours, and carcinoma ex pleomorphic adenoma (CXPA) 10–15 %; polymorphous low-grade adenocarcinoma (PLGA) also approximately 10–15 %; acinic cell carcinoma (ACC) somewhat less frequent, approximately 10 % (but ACC is the second most common malignancy in major salivary glands, approximately 15 %); and salivary duct carcinoma (SDC) 5–10 %. Hence MEC, ADCC, CXPA, PLGA, ACC and SDC together constitute the vast, vast majority of salivary gland malignancies, some 85–95 % [27, 30, 56, 85, 182, 189]. Mucoepidermoid carcinoma is not only the most common salivary gland malignancy, but it is also the most common malignant salivary gland tumour in children and the most common of all malignant intraoral salivary gland tumours. Mucoepidermoid carcinoma is the most common salivary malignancy arising in survivors of childhood cancer that initially were treated with a combination of radiation and chemotherapy [6, 58, 180, 186]. A disproportional high frequency of mucoepidermoid carcinomas has been reported amongst atomic bomb survivors suggesting a causal role for ionising radiation for mucoepidermoid carcinomas in contrast to other salivary gland malignancies. Mucoepidermoid carcinomas have been reported to arise as a secondary malignancy within a year as well as after 21 years in patients treated with chemotherapy and chemoradiotherapy, respectively, for other malignancies [23, 190]. The use of mobile phone and its association with different health disorders have been a much debated issue, particularly perhaps brain tumours and the long-term risk for hearing problems. A recent study from China studied any possible association between the use of mobile phone and parotid epithelial malignancies. The study comprised 136 patients with epithelial parotid malignancies and 2,501 controls without tumours, all during a 17-year period from 1993 to 2010. Their findings indicated that the frequency of mobile phone use was not significantly associated with parotid malignancy [52].
7.2 Definition, Site and Incidence
Mucoepidermoid carcinoma (MEC) presents a wide variety of histological appearances. It is defined as a tumour comprising a mixture of mucus-secreting cells, epidermoid cells and cells of intermediate type, present in varying proportions. There may also be clear cell and oncocytic features present. It was previously suggested that the epidermoid and mucous cells of mucoepidermoid carcinoma arise from an undifferentiated cell associated with the excretory ducts. However, experimentally many cells from different salivary gland epithelia, even acinar cells, are found to be capable of proliferation. The apparent absence of myoepithelial cells in MEC casts doubt on the concept of segregation of MEC from the many other entities said to originate from the intercalated duct reserve cell. It has been proposed that the intermediate cells of mucoepidermoid carcinoma are the counterpart of the modified myoepithelial cells of pleomorphic adenoma [45]. The apparent lack of positivity for myoepithelial markers with only scattered positivity for p63 does not support the intermediate cell to be myoepithelial in nature. Since all parts of the duct system have luminal and myoepithelial or basal cells, MEC as well as other neoplasms could develop from a variety of cell types in any segment of the ducts and yet have similar histological appearances. It is more likely that the intermediate cell differentiates from striated duct cell components. The vast majority of mucoepidermoid carcinomas arise de novo but may occasionally arise in a pre-existing benign salivary gland tumours, e.g. from pleomorphic adenoma and Warthin tumour. Early attempts were made with some success to classify MEC into different prognostic groups, but some denied the malignant nature of the lowest grade. This led to the whole entity being referred to as mucoepidermoid ‘tumour’, a nomenclature that unfortunately was validated by the first WHO classification in 1972. The term mucoepidermoid carcinoma was restored in the second WHO classification in 1991 and is now widely accepted [71, 142].
Approximately 50 % of mucoepidermoid carcinomas arise in the major salivary glands and the vast majority of the remaining cases in the oral cavity, particularly the palate and buccal mucosa. It is the most common salivary gland malignancy irrespective of the site (except the sublingual and lacrimal glands). In the lacrimal gland, MEC is the second most common malignant salivary-type tumour after adenoid cystic carcinoma [130]. It is approximately six times more common in the parotid than in the submandibular gland. Only 1–2 % of mucoepidermoid carcinomas arise in the sublingual gland. Mucoepidermoid carcinoma is the most common malignant intraoral salivary gland tumour, and only pleomorphic adenoma is a more common intraoral salivary neoplasm. Mucoepidermoid carcinoma constitutes approximately 20 % of all intraoral salivary gland tumours and 50 % of all malignant intraoral salivary tumours, whereas the main proportion of other malignant intraoral salivary tumours are adenoid cystic carcinomas (25 %) and polymorphous low-grade adenocarcinomas (20 %) [30, 54, 100, 189]. Centrally intraosseous occurring mucoepidermoid carcinoma is rare with some 100 cases described in the literature, many of which in teenagers. The vast majority arise in the mandible, and only a very few exceptional cases have been described in the maxilla [38, 43, 68, 87, 93, 119, 144, 145, 152, 169]. Mucoepidermoid carcinomas of the larynx, nose and sinuses have also been described [47, 120, 167]. Less than 25 cases have been described in the nasopharynx, and they are also exceedingly rare in the external auditory canal. Its existence in the middle ear is controversial [11, 92, 133]. Mucoepidermoid carcinoma may arise in organs other than the salivary glands, e.g. breast, oesophagus, pancreas, lung, skin, thymus and thyroid. Recently a low-grade variant of pulmonary mucoepidermoid carcinoma with prominent tumour-associated lymphoid proliferation has been reported [148].
Thymic mucoepidermoid carcinoma is rare and is one of the most unusual forms of at least ten different existing histological variants of thymic carcinoma. It consists, like its salivary gland counterpart, of a mixture of squamous, mucous and intermediate cells. Thymic mucoepidermoid carcinomas are predominantly of low-grade malignancy and may also be associated with multilocular thymic cysts [31, 115, 121, 134, 157, 160, 168].
Two types of thyroid mucoepidermoid carcinoma have been described, conventional mucoepidermoid carcinoma and sclerosing type of mucoepidermoid carcinoma. The latter is usually associated with eosinophilia, but there are also cases of thyroid sclerosing mucoepidermoid carcinoma without eosinophilia. Most of the cases reported have been of low-grade malignancy. Sclerosing mucoepidermoid carcinoma with eosinophilia is characterised by a mucoepidermoid carcinoma with extensive sclerosis and a concomitant inflammatory infiltrate rich in eosinophils. The uninvolved thyroid tissue usually shows lymphocytic thyroiditis. Immunohistochemical findings suggest that these two types may have different histogenesis, the conventional mucoepidermoid carcinoma being positive for thyroglobulin whilst the sclerosing variant is negative. Studies have also showed sclerosing mucoepidermoid carcinoma to be positive for p63 which is a marker of remnants of the ultimobranchial body (UBB, solid cell nests). It therefore may seem likely that the sclerosing variant arises from UBB/solid cell nests rather than follicular epithelial cells [1, 10, 33, 59, 78, 151]. There are findings, however, that suggest that primary thyroid mucoepidermoid carcinoma derives from dedifferentiation of thyroid follicular cells. Tumours were immunohistochemically negative for thyroglobulin and calcitonin, but RT-PCR investigation showed presence of thyroid-specific genes as TTF-1, TTF-2, Pax-8, NA-I symporter and thyroid peroxidase mRNA [112].
Mucoepidermoid carcinomas show a slight female predilection which may however vary according to the anatomical site of the tumour. For example, mucoepidermoid carcinomas of the tongue have a higher female/male ratio than the general female predominance of 3:2. Mucoepidermoid carcinomas appear in all ages, and very much so also in children being the most common malignant salivary tumour in children, but have rather uniform age distribution with diminution at both ends of the age spectrum [71].
7.3 Clinical Features and Histopathology
Mucoepidermoid carcinomas of the major salivary glands usually present as painless swellings and are often firm but variably movable or fixed. Neural invasion occurs and pain as well as facial paralysis may be early symptoms. Anecdotally, in a unique case of tongue mucoepidermoid carcinoma, the neural invasion caused myokymia (fasciculation) of the tongue rather than destruction of the nerve [4]. Minor salivary gland MECs also typically appear as asymptomatic swellings. They may have a red or bluish colour and be fluctuant and therefore can be mistaken clinically for a mucocele, particularly when located in the lip. Intraosseous mucoepidermoid carcinomas usually present as a cortical swelling with radiographs revealing uni- or multilocular radiolucency with well-defined borders. It may be associated with an unerupted tooth and therefore mimic an odontogenic cyst. The interval between initial swelling and histological diagnosis is commonly less than 1 year. Some of the high-grade tumours manifest themselves earlier whilst the interval between initial swelling and diagnosis for some of the low-grade tumours may be as much as 5–6 years. Parotid tumours are usually 1–2 cm but may be as large as 4 cm, and occasional long-standing but high-grade tumours are larger than 5 cm. Mucoepidermoid carcinomas are nonencapsulated neoplasms and mostly poorly circumscribed. They tend to be firm but are also often cystic and have a white or pinkish cut surface (Fig. 7.1).
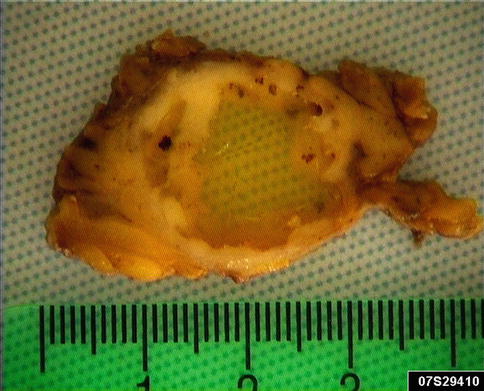

Fig. 7.1
Cystic mucoepidermoid carcinoma that presented as persistent slowly growing parotid swelling for years
Mucoepidermoid carcinoma is a heterogeneous tumour histologically composed of three distinctive cell types, namely, epidermoid, mucous and intermediate cells. Hydropic degeneration and metaplastic changes occur inducing the presence of clear, columnar and oncocytic cells. The tumours can be solid, but most often they have cystic spaces of varying sizes, the contents of which stain positively with PAS, with and without diastase, alcian blue and mucicarmine. The mucous cells have a sialomucin which is best demonstrated by mucicarmine or alcian blue staining. The sialomucin content is almost totally lost in the degenerated clear cells, which, however, are diastase-sensitive PAS positive indicating presence of glycogen (Fig. 7.2). The cystic spaces are primarily lined with columnar or cuboidal mucous cells, but there are also numerous interspersed cells of basaloid intermediate type as well as a few epidermoid cells. The mucous cells lining cysts can be a single layer or multilayered (Fig. 7.3). The mucous cells are columnar or cuboidal goblet cells with often pale, eosinophilic, foamy cytoplasm that may resemble normal acinar cells. When mucous cells are sparse, or not well differentiated, visualisation of mucin by special stains can be diagnostic for a MEC. Occasionally the mucous cells acquire a signet ring cell appearance.
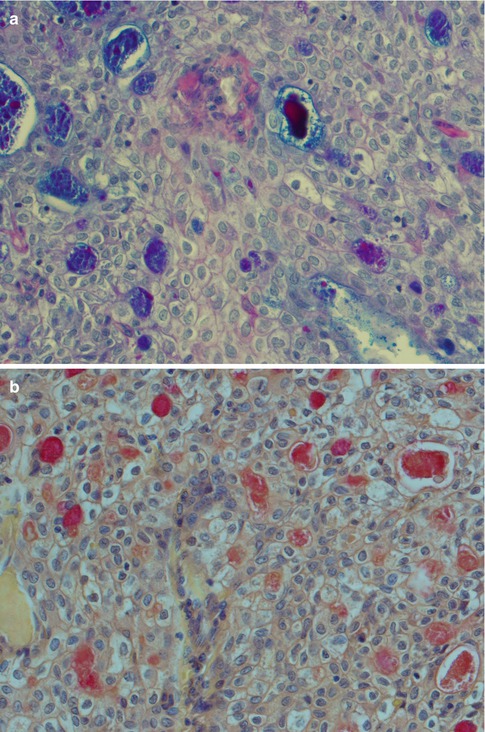
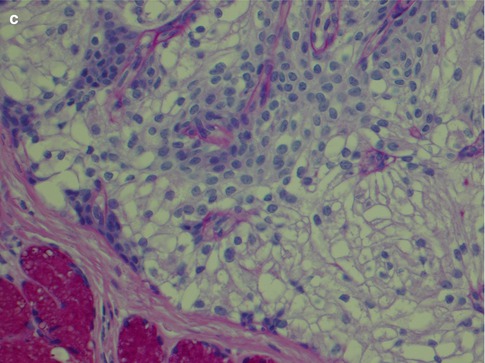
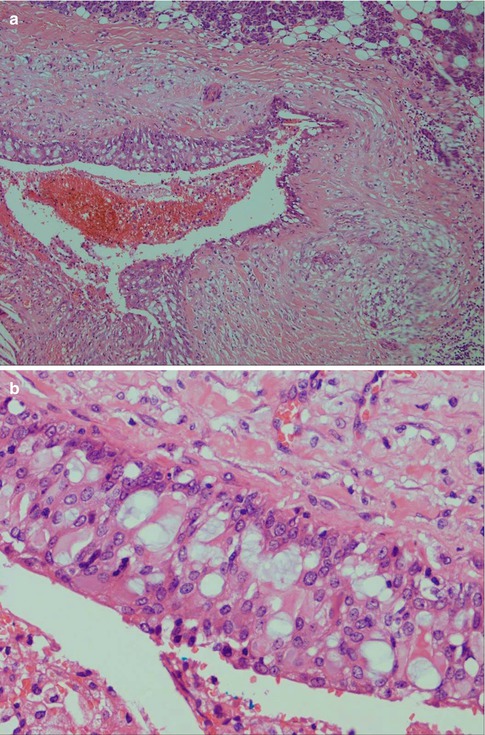
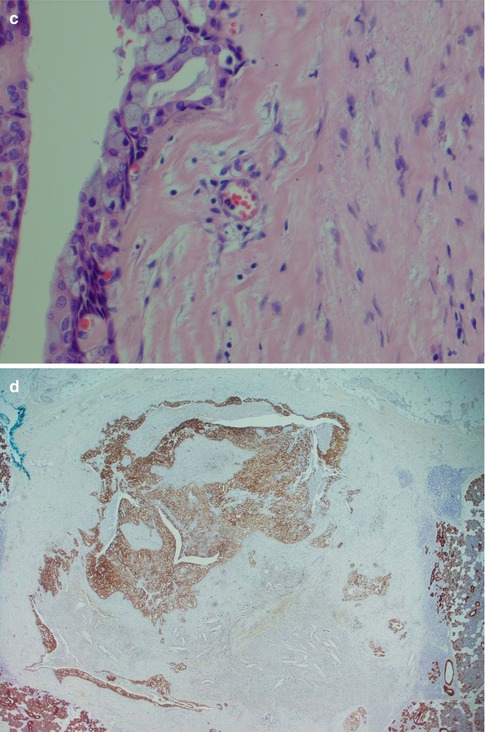


Fig. 7.2
(a) AbPAS staining of a low-grade mucoepidermoid carcinoma with intracellular sialomucin as well in the cystic spaces. (b) Mucicarmine stain of the same tumour. (c) Clear cells show loss of PAS staining after diastase treatment


Fig. 7.3
(a) Cystic low-grade MEC. (b) Higher magnification showing the cyst lining with numerous mucous cells and interspersed basal intermediate cells. (c) Lining of cystic space with numerous mucous cells, a few epidermoid cells (bottom) and above the latter some intermediate cells. (d) Cytokeratin stain illustrating the invasive nature of a low-grade MEC
The intermediate cells are smaller than the mucous cells and are in the cyst linings often situated below the mucous cells. They range in size and appearance from small basaloid cells to almost epidermoid in appearance. In the transition between these two cell types, there are more eosinophilic cells that gradually have accumulated additional cytoplasm and more easily delineated. They have sharply demarcated cytoplasmic borders and the nuclei are usually dark staining. When the intermediate cells are arranged in nests and sheets in a syncytial arrangement, the nuclei may acquire a vesicular appearance (Fig. 7.4). Some reports indicate that intermediate cells stain strongly for low molecular weight cytokeratins whilst the epidermoid cells stain more strongly with high molecular weight cytokeratins. I find the difference in staining is rather subtle and of little practical use. The intermediate cells are by some regarded as the counterpart of modified myoepithelial cells of pleomorphic adenoma [45], but they stain negative for markers indicative for myoepithelial differentiations, for example, smooth muscle actin, h-caldesmon and smooth muscle myosin heavy chain, and only very occasionally are positive for S-100. Myoepithelial cell differentiation in mucoepidermoid carcinoma is hence limited to rare cells, and it appears more likely that the intermediate cells differentiate from striated duct cell components, i.e. from the columnar, eosinophilic and mitochondria-rich columnar striated duct cell and/or the basal cell [62]. The frequently found strong positivity for CK7 in MEC would also support an origin from columnar striated duct cell components. The intermediate cell is the most difficult to recognise of the three cell types in a mucoepidermoid carcinoma, but its demonstration can be important in the differential diagnosis between a mucosal MEC and adenosquamous carcinoma.
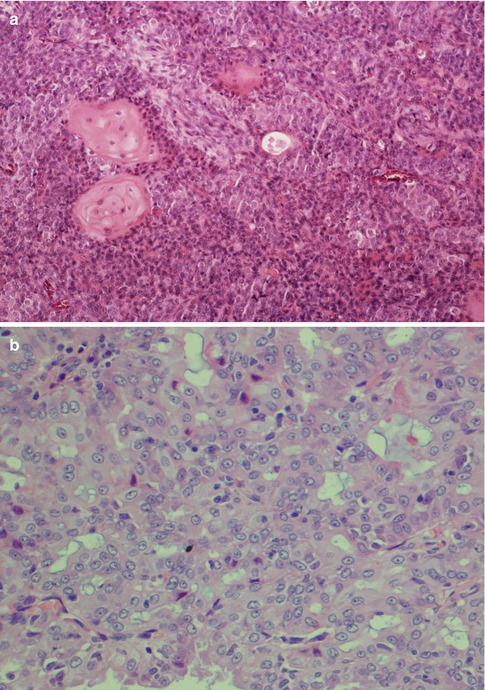
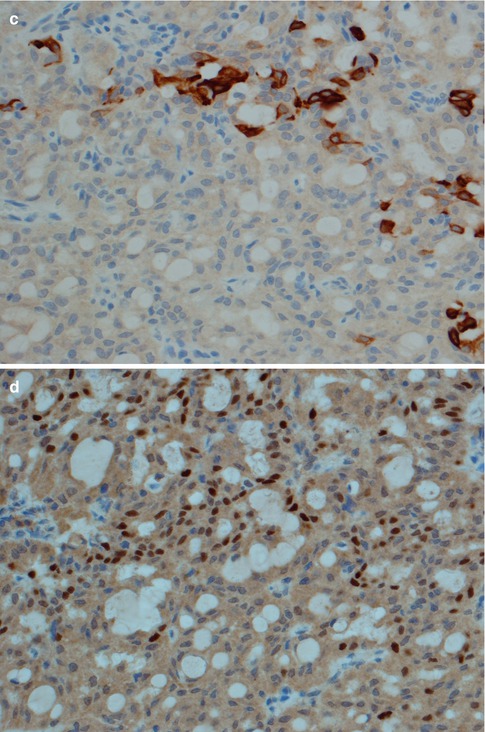


Fig. 7.4
(a) Typical intermediate cells with small dark nuclei and some foci with epidermoid cells. (b) Another tumour with intermediate cells arranged in solid nests, almost syncytial arrangement, and where many nuclei appear somewhat vesicular. (c). The same tumour as in (b) with CK14 positivity restricted to mucous and epidermoid cells. (d) Same tumour with scattered positivity for p63
The epidermoid cells are larger, round to ovoid cells with abundant eosinophilic cytoplasm. When enlarged they eventually acquire a polygonal cell outline and may appear as mature squamous cells. They show intercellular bridges but only occasionally keratinisation and individual keratin pearls. Oncocytic metaplasia is rare in mucoepidermoid carcinoma, but hydropic degeneration with the development of areas of clear cells is rather common and may even be a dominant feature and in those cases referred to as clear cell or oncocytic variant of mucoepidermoid carcinoma. The clear cells tend have a large polygonal shape with sharply demarcated borders and have small nuclei, often pyknotic and centrally placed. The clear cells have minimal sialomucin but are diastase-sensitive PAS positive, hence implying glycogen content. On rare occasions, dystrophic calcifications have been noticed in MED with clear cells [51, 53, 73, 82, 188]. A recent study does however indicate that calcifications may be a frequent finding in MECs and not as rare as previously believed. Calcifications were noted in 6 of 30 cases of MEC (20 %) [69]. An abundant lymphoid stromal component is not unusual, particularly as an inflammatory infiltrate adjacent to more cystic variants of mucoepidermoid carcinoma (Fig. 7.5). More rarely pigmented melanocytes can be present in the stroma of mucoepidermoid carcinoma [143]. Spindle- and dendritic-shaped cells containing melanin are not a feature unique for MEC but can also be seen in, for example, pleomorphic adenoma and adenoid cystic carcinoma. Melanin granules have also been described in the epithelial cells of MEC and thought to represent phagocytosis by the neoplastic cells [106].
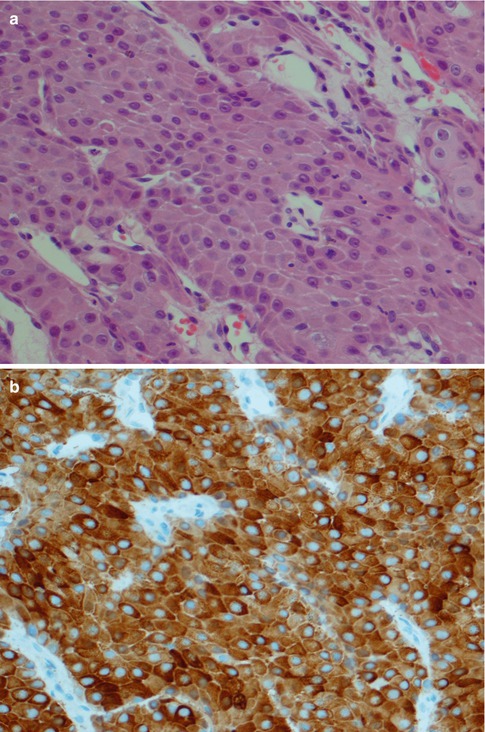
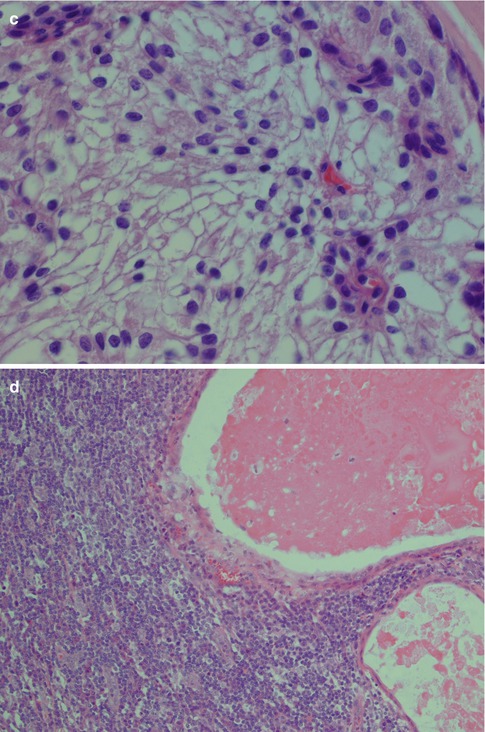


Fig. 7.5
(a) Epidermoid cells with intercellular bridges but no keratinisation. (b) Strong positivity for high molecular weight cytokeratins. (c) Clear cells with sharply delineated borders and small nuclei. (d) Cystic mucoepidermoid carcinoma with a dense surrounding inflammatory infiltrate
Since the accurate original description of mucoepidermoid carcinoma in 1945 by Stewart and associates [164], several systems have been proposed to grade this neoplasm. The previous 1991 WHO classification [142] recommended two grades, low- and high-grade carcinomas, whilst the current 2005 WHO classification appears to favour a three-level grading. It is however emphasised that none of the many classifications has been universally accepted over the years. A three-tiered histological grading was proposed by Healey and associates in 1970 [75] and later modified by Batsakis and Luna in 1990 [12]. It was used in several clinicopathological studies [42, 77], particularly after it had gained a wider acceptance in 1992 after the first AFIP grading proposal [8]. The AFIP system was refined in 1998 by a weighted AFIP histological grading schema [72]. A modification of the weighted AFIP system by the addition of more histological criteria was proposed in 2001 [25]. Regardless if the grading is done into two or three grades, the grading is based on the prevalence of the different cell types, i.e. mucinous, intermediate and epidermoid cells, and histological features of aggression, e.g. cellular and nuclear polymorphism, vascular invasion, necrosis and high mitotic count. Briefly, low-grade tumours contain a prominent mucinous component, often well-circumscribed tumour nests with pushing borders. They contain cystic spaces that often are lined with columnar cells and have low mitotic activity and little cellular atypia. Intermediate-grade tumours have less cysts and less mucinous cells and have a prominent population of intermediate cells. The cell nests are more irregular than in low-grade tumours, and cellular atypia is more pronounced. High-grade tumours are more solid, contain more epidermoid cells and display cellular anaplasia, necrosis and a high mitotic count. The weighted AFIP grading system is based on a point score for each of the five histological features. An intracystic component <20 % and neural invasion were given a point value of 2 each, whilst necrosis and high mitotic count (4 or more per 10 HPF) were given a point value of 3. Presence of cellular anaplasia as characterised by cellular and nuclear pleomorphism was given a point value of 4. Tumours with a total point score of 0–4 were classified as low grade and 5–6 as intermediate grade, and tumours with point score 7–14 were graded as high-grade tumours [72]. The modified AFIP system proposed by Brandwein and associates in 2001, and also recently used in a multi-institutional review [9, 25], further included histological features as tumour front invasion by small nests and islands, vascular invasion and bone invasion. The three-level system has a better bearing on prognosis than the two-level system and also clearly recognises the intermediate cell population as an integral histogenetic and histological component. Further, the three-grade system better separates out the poorly differentiated type associated with a dismal prognosis. In summary, the Brandwein’s system upgrades tumours for lack of cystic content alone, whereas the AFIP system allows for minimal cyst content whilst still being low grade. Further, the Brandwein’s grading system takes into account the nature of the invasive front of the tumour. Still there are rather strong arguments in favour of using the two-level grading system. Mucoepidermoid carcinoma is the most common malignant salivary gland tumour, but nevertheless it is a rare neoplasm. A medium- to large-sized laboratory (40–60,000 surgicals/annum), likely serving a population of 800,000–1,000,000 inhabitants, will still not encounter more than half a dozen cases per year. Such a laboratory would likely by staffed by at least a dozen of pathologists, and therefore a mucoepidermoid carcinoma does not come across to the individual pathologist that often. Even in laboratories with subspecialisation, the head and neck/oral pathologist will not see more than many cases per year. Hence it will be difficult and take long time to achieve any substantial personal experience of these tumours. Without a solid personal experience, we believe it would be very difficult to use the three-level grading system with an acceptable degree of reproducibility. For pathologists working at large referral centres, the situation is of course different. Another argument against the use of the three-level system in routine pathology outside head and neck oncology centres is that the intermediate-grade tumours most often still would have to be treated as high-grade tumours (possible lymph node exploration, possible adjuvant radiotherapy/chemotherapy) and thus in these settings diminishing the clinical value of a three-level histological grading. There are hence several reasons to why the two-level grading is more practical to use and it is likely wiser for the general pathologist to adhere to the two-level grading system recommended by the WHO classification of 1991 [142]. On rare occasions low-grade MEC may however, as well as other low-grade malignancies, e.g. acinic cell carcinoma, epithelial-myoepithelial carcinoma and polymorphous low-grade adenocarcinoma, dedifferentiate or transform into a high-grade neoplasm [3, 50, 61, 116, 140, 153]. The subject of high-grade transformation, not only in low-grade malignancies, is further discussed in Chap. 16.
Low-grade mucoepidermoid carcinomas (or well-differentiated MECs) are partly circumscribed but nonencapsulated tumours. They are usually less than 3–4 cm in diameter and considerably smaller in minor salivary glands. They are predominantly cystic with larger cysts as well as numerous smaller cystic spaces. The larger cysts are typically lined with columnar cells and interspersed mucus-secreting cells and especially so in minor salivary gland MECs. The cystic areas occupy at least 20–25 % of the tumour (Figs. 7.2 and 7.6). Low-grade MECs contain a prominent population of mucous cells but also many epidermoid cells, and most of them are well differentiated. There is intracellular mucin as well as mucous present in the cystic spaces. There are also usually many foci with clear cells, some of which contain mucin whilst other clear cells are mucin-negative but glycogen-positive (Fig. 7.2). The intermediate cell type is usually below the mucous cells in the cyst lining or interspersed amongst the mucous and epidermoid cells. However, some tumours have large sheets of intermediate cells, and these tumours would often have been graded as intermediate in a three-level grading system. Low-grade tumours tend to invade by pushing margins with smooth cohesive tumour islands (Fig. 7.7). Many may however have more aggressive tumour infiltration with small islands and individual cells. In the latter cases, the lack of cellular anaplasia and low mitotic count will determine the classification as low-grade tumour. Low-grade mucoepidermoid carcinomas can have a rather pronounced surrounding inflammatory infiltrate. The inflammatory infiltrate is primarily found around the cystic areas but can also be seen around solid nests of cells and in those cases often of nests consisting of intermediate cells. High-grade nuclear atypia is not a feature of low-grade mucoepidermoid carcinoma, and the mitotic activity is usually low. Mitoses are absent or rare and there may be 1–2 mitoses per HPF. Three to four mitoses per HPF is the cutoff point for high-grade tumours. Necrosis, perineural and vascular invasion are not features of low-grade mucoepidermoid carcinomas.
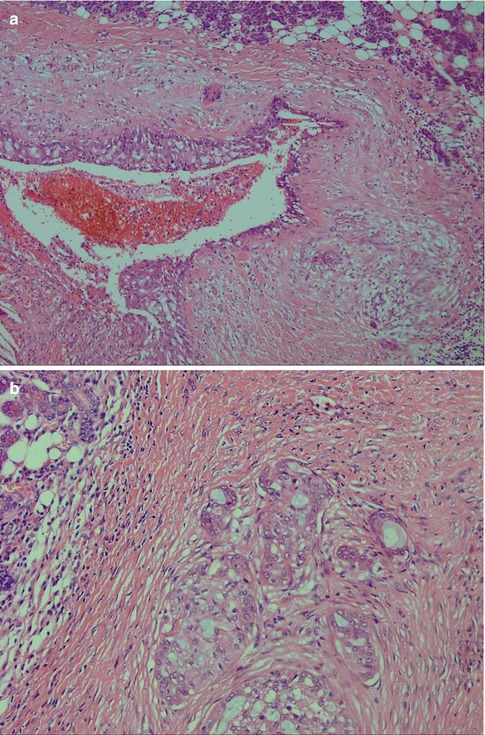
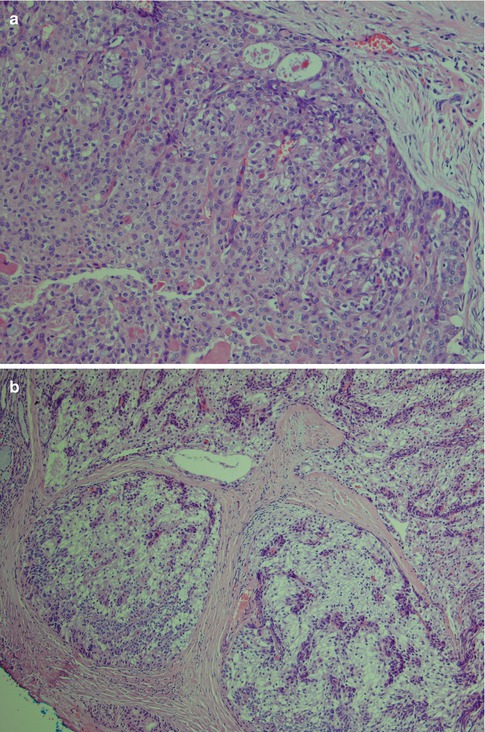
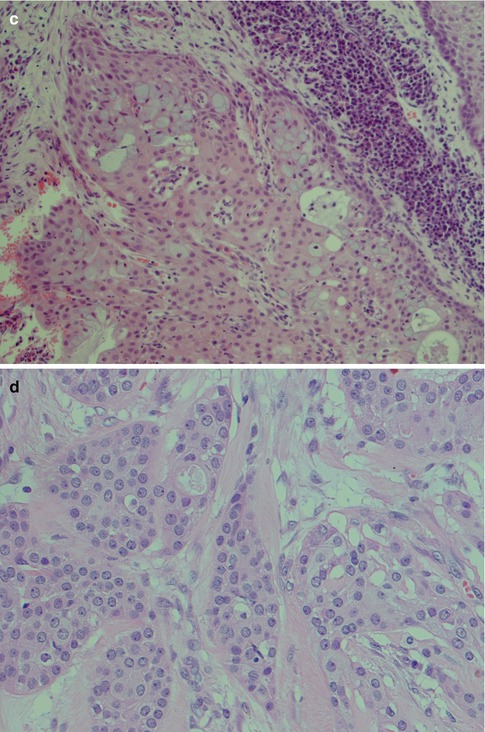
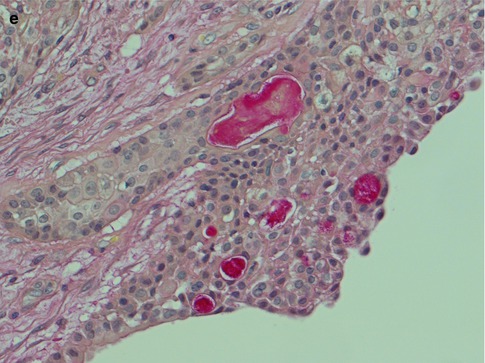

Fig. 7.6
(a) Small cystic parotid low-grade MEC. (b) Areas with infiltrative growth and a desmoplastic stroma



Fig. 7.7
(a) Solid part of a low-grade MEC with pushing infiltrative margins. (b) Low-grade clear cell variant with blunt, rounded infiltrative borders. (c) In spite of a large proportion of epidermoid cells and not so many mucous cells (upper part of picture), the lack of cellular atypia and pushing margins determine this tumour as of low-grade malignancy. Also note the surrounding lymphoplasmacytic infiltrate. (d) Epidermoid cells with little atypia. (e) Low-grade cystic MEC with interspersed mucous cells (mucicarmine)
High-grade mucoepidermoid carcinomas are considerably less common than the low-grade tumours. Compiling data from different series of mucoepidermoid carcinomas, low-grade tumours appear approximately four times more common than high-grade mucoepidermoid carcinomas. High-grade MECs are composed mainly of epidermoid cells and intermediate cells, often arranged in compact nests. The most important single histological features are cellular anaplasia, necrosis and high mitotic count. Cellular anaplasia is defined as nuclear polymorphism and increased nuclear-cytoplasmic ratio, large prominent nucleoli, anisochromia and hyperchromasia (Fig. 7.8). Vascular and neural infiltration are features of high-grade tumours, albeit not so common, but indicative of very poor prognosis. In most cases, the infiltrative cells are the epidermoid cells and occasionally the intermediate cells. Vascular invasion by mucous cells is rarely, if ever, seen. The neural infiltration tends to be perineural rather than intraneural (Fig. 7.9). Cystic spaces are present in high-grade tumours but considerably less in numbers, and they are also smaller than those seen in low-grade tumours. Cystic spaces typically comprise less than 20 % of a high-grade tumour. High-grade tumours usually show an aggressive infiltrative margin with single or small nests of atypical cells (Fig. 7.10). Mucous cells are present but are relatively sparse and constitute usually less than 10 %, and in many such cases, a special mucin stain such as mucicarmine or PAS is needed to visualise the sparse mucous cells. These tumours have a high proportion of epidermoid cells, and particularly in the parotid gland, they can have strikingly similar histological features to a metastatic, moderately well-differentiated squamous cell carcinoma. Mucoepidermoid carcinomas are immunopositive for CK7 and BER-EP4, features that rarely, if ever, are shared by metastatic skin squamous cell carcinomas (Fig. 7.11). High-grade mucoepidermoid carcinomas often have irregular islands of epidermoid and intermediate cells with vesicular nuclei and often prominent nucleoli. There are smaller cysts, sometimes filled with mucin, and sometimes glandular formations but mucinous cells are not conspicuous. There can be a prominent fibrous, almost sclerotic reaction with chronic inflammation partly mimicking a salivary duct carcinoma. Foci of necrosis and perineural invasion are not uncommon, whilst vascular invasion is seen less frequently (Fig. 7.12). A summary of histological features distinguishing low-grade from high-grade mucoepidermoid carcinomas is given in Table 7.1.
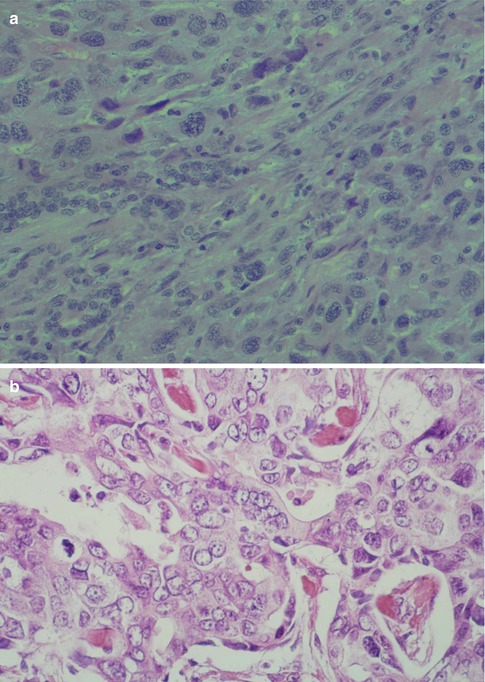
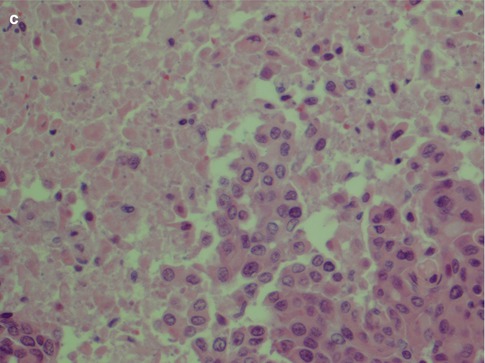
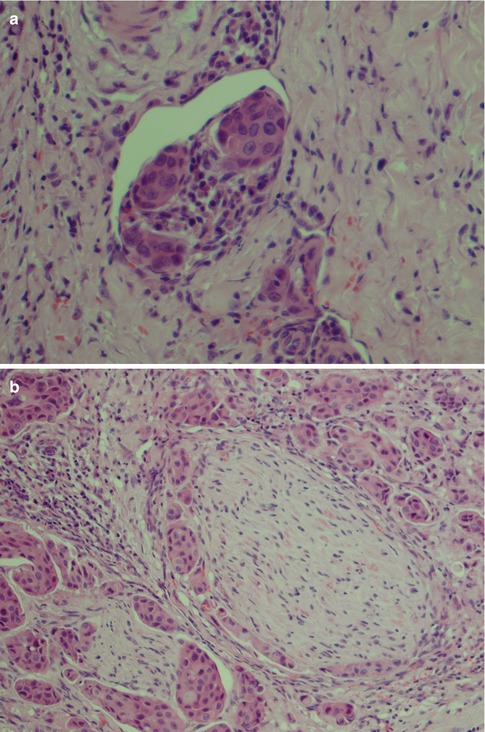
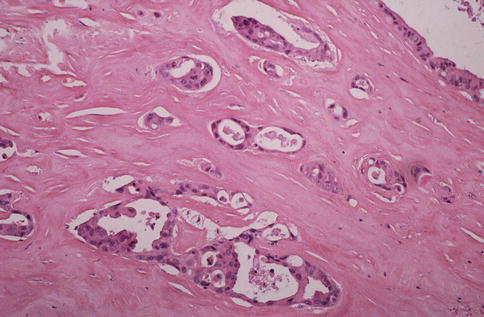
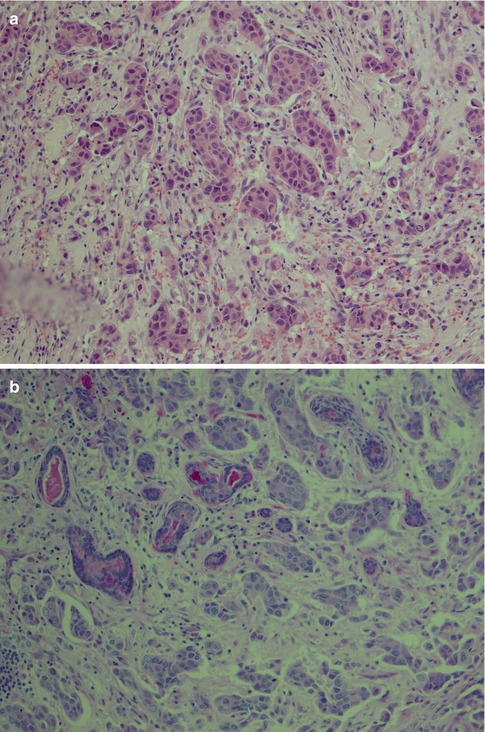
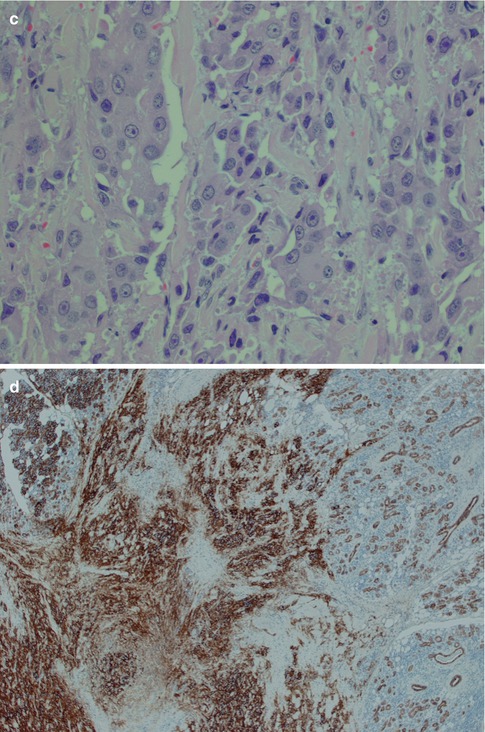
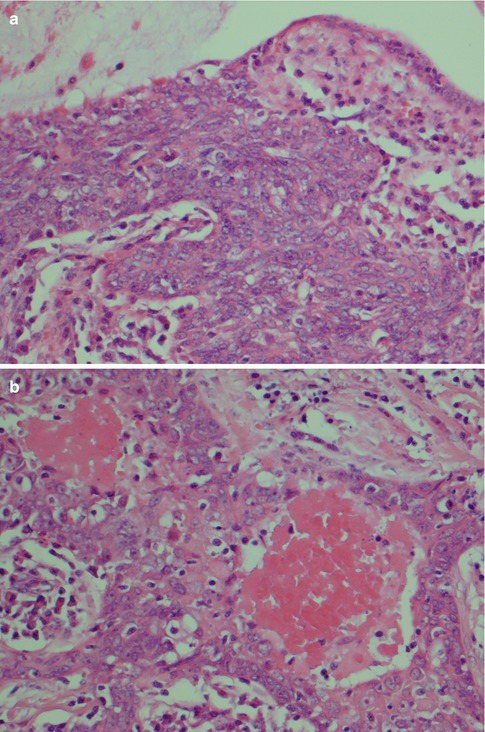
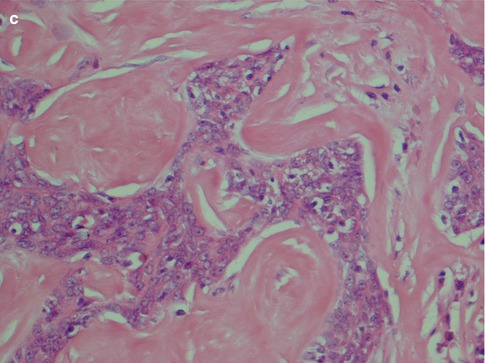


Fig. 7.8
High-grade MEC. (a) Pronounced cellular anaplasia with bizarre cells with hyperchromatic nuclei. (b) Severely atypical cells with prominent nucleoli and atypical mitoses. (c) Area with necrosis surrounded by epidermoid tumour cells

Fig. 7.9
(a) Vascular infiltration of epidermoid tumour cells. (b) Perineural infiltration of predominantly epidermoid cells

Fig. 7.10
Cystic high-grade MEC with infiltration of small groups of cells as well as single cells. The cells are too atypical compared to those of low-grade tumours


Fig. 7.11
(a) High-grade tumour consisting of sheets and nest of primarily epidermoid cells. (b) Only few PAS-positive lumina can be detected in the same tumour. (c) Parotid high-grade MEC with epidermoid-like cells. The nuclei are vesicular with prominent nucleoli. (d) The same case with positive immunostain with BER-EP4 which highlights the infiltrative pattern and excludes metastatic skin squamous cell carcinoma


Fig. 7.12
High-grade parotid MEC. (a) Cystic space with mucin (top) and solid sheets of atypical cells. (b) Foci of necrosis. (c) Tumour islands cuffed by hyalinised, almost sclerotic stroma
Table 7.1
Guidelines for histological grading of mucoepidermoid carcinoma
Low grade | High grade |
|---|---|
<4 cm | >4 cm |
May be circumscribed but not encapsulated | Poorly defined margins |
Predominantly cystic | Predominantly solid |
Cystic spaces occupy at least 20–25 % | Cystic spaces occupy clearly <20 % of the tumour |
>50 % of cells are well-differentiated mucous and epidermoid cells | <10 of cells are mucous cells |
Pushing margins | Infiltrative margins |
Often prominent surrounding inflammatory infiltrate | Fibrosis, sclerosis (may mimic salivary duct carcinoma) |
<1–2 mitoses/HPF | >3–4 mitoses/HPF |
No high-grade cellular anaplasia | High-grade cellular anaplasia |
No necrosis, haemorrhage or vascular invasion | Necrosis, haemorrhage and perineural invasion not uncommon, vascular invasion less common |
HER2 negative | Occasionally HER2 positive |
Mucoepidermoid carcinoma is well known to be a morphologically heterogeneous entity (as well as genetically; please see below, Sect. 7.6) with many mimics (please see below, Sect. 7.5). In a series of 40 MECs, 23 presented with classic morphology, and 9 had predominant squamoid features, 5 eosinophilic/oncocytic and 3 clear cell morphology [138]. There are also cases of mucin-rich MECs which currently are regarded to belong to a group of mucin-producing salivary carcinomas (mucinous cystadenocarcinoma, colloid carcinoma, mucin-rich salivary duct carcinoma and mucin-rich MEC) [187]. In spite of the above, there are currently only two variants of MEC that are recognised as distinct entities, namely, the oncocytic and the sclerosing variants. Oncocytic mucoepidermoid carcinoma is a rare variant of MEC with only approximately 50 cases described in the literature so far. Most of these cases have been published as rare case reports until the clinicopathological study of 12 cases by Weinreb and associates in 2009 [185]. Oncocytic MEC is characterised by an extensive presence of oncocytic cells, usually 60–70 % of the neoplasm. These tumours are low-grade MEC, but with such prominent oncocytic change, they may be confused with oncocytic tumours (see below, Sect. 7.5, and Chap. 15). Most oncocytic MECs have been reported from the parotid gland [26, 40, 48, 60, 73, 82, 150, 179, 185] but also some from the submandibular gland [44, 90], sublingual gland [185], trachea and bronchus [98, 159], palate [46, 105, 185] and lacrimal gland [96, 132].
As previously discussed, sclerosing mucoepidermoid carcinoma with or without eosinophilia (SMEC) exists in the thyroid gland but is present in both major and minor salivary glands. It is rare and so far only some 50 cases have been reported since it was discovered in 1987 [32, 57, 76, 79, 111, 149, 154, 170, 175, 177]. SMEC is characterised histologically by dense stromal sclerosis and lymphoplasmacytic infiltrates, with or without an eosinophilic infiltrate. It has been debated whether it represents a distinctive tumour entity, but at present the prevailing view is that it is a variant of conventional mucoepidermoid carcinoma. Its presence in the salivary gland tissue further supports the concept that SMEC with or without eosinophilia develops from some basal type cells, common in both salivary and thyroid tissue, rather than thyroid follicular cells. Tumour infarction and extravasation of mucin with reactive fibrosis are other possible mechanisms of formation that have been suggested as underlying this histological (sub)type of mucoepidermoid carcinoma. Still, these morphological events require a pre-existing MEC. In a study by Tasaki and associates, reverse transcription PCR was used, but the fusion gene transcripts that currently are regarded as specifically identified with mucoepidermoid carcinoma were not detected in their case of salivary SMEC [170]. Another recent study by Tian and associates interestingly demonstrated that in their series, albeit a small series of six patients with SMEC, the number of IgG4-positive plasma cells was significantly increased compared to non-sclerosing MECs. None of these six patients had IgG4-related sclerosing disease (see also Chap. 2), and the finding of this study hence suggests a role of IgG4 plasma cells in the fibrinogenesis in SMEC [171].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


