and Aysegul A. Sahin2
(1)
Division of Pathology, Singapore General Hospital, Singapore, Singapore
(2)
The University of Texas, M. D. Anderson Cancer Center, Houston, TX, USA
Keywords
Mucinous metaplasiaCystMucocele-like lesionMucin extravasationSolid papillary carcinomaNeuroendocrineMucinous breast lesions represent a heterogeneous group of entities that are described together based on the presence of predominantly extracellular mucin as a common feature. Intracytoplasmic mucin may be seen rarely as part of benign metaplasia, but are more frequently observed in invasive and in situ carcinomas [1]. Mucinous breast lesions listed in the chapter range from benign conditions like cysts and dilated ducts with luminal mucin to mucocele-like lesions that can be associated with a range of epithelial proliferations, and to mucin-producing malignant neoplasms of mucinous carcinoma and solid papillary carcinoma with mucin production.
Cysts with Luminal Mucin and Mucinous Metaplasia
Definition
Cysts of varying sizes, commonly encountered in breast biopsies, comprise dilated ducts within fibrous parenchyma. While microscopic breast cysts are commonly encountered incidentally on histological assessment of breast tissue, duct dilatation can be minimal to significant, forming cysts that may be clinically symptomatic. These cysts often contain luminal secretions which are granular and eosinophilic. On occasion however luminal mucin which distends the ducts, may be observed.
Clinical and Epidemiological Features
Mild cystic changes of the breast are asymptomatic. The exact incidence of breast cysts is unknown; they are likely to be widely prevalent. When cystic dilatation is marked, patients may present with mastalgia, breast lumps, or lumpiness. Luminal mucin in ducts has been reported in 6 % of normal breasts, compared to its occurrence in benign ducts of 80 % of breasts containing mucinous carcinoma [2].
Imaging Features
Breast cysts can be seen on imaging. Scattered or loose groups of luminal microcalcifications are commonly observed on mammography. In particular, layering of calcium in microcysts giving rise to a teacup appearance on mammography is unique to cystic changes in the breast (Fig. 6.1).


Fig. 6.1
Mammographic appearance of breast cysts. Magnification view in the true lateral projection shows teacups or layering of calcifications in the dependent aspect of microcysts (Courtesy of Dr. Lester Leong)
Pathologic Features
Macroscopic Pathology
Breast cysts may be macroscopically occult. They are often associated with fibrous areas in the breast parenchyma within which are scattered small cysts (Fig. 6.2).


Fig. 6.2
Breast cystic change. Gross appearance shows ill-defined fibrous areas with scattered small cysts
Microscopic Pathology
Breast lobules show variable dilatation of ducts and acini within fibrous stroma. Luminal mucin can occur as small amounts of wispy, acellular material within dilated ducts or as more abundant mucin that distends ducts (Fig. 6.3). Calcifications may be observed within the mucin (Fig. 6.4). When there is marked ductal distension with rupture and extrusion of mucin into surrounding stroma, the diagnosis of mucocele-like lesion is appropriate.
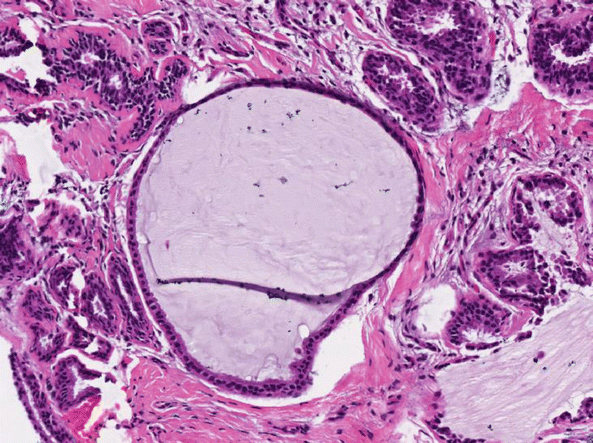


Fig. 6.3
Breast cyst with luminal mucin. Cystically dilated ducts contain luminal mucin. Although the lining of one of the affected ducts appears to be incomplete, no convincing extrusion of mucin into the surrounding stroma is seen. If there is mucin extravasation, the diagnosis of mucocele-like lesion is appropriate

Fig. 6.4
Cystically dilated ducts contain luminal mucin and calcifications
Mucinous metaplasia is an uncommonly encountered microscopic alteration in the breast, in which lining epithelial cells show mucinous cytoplasm (Figs. 6.5 and 6.6).



Fig. 6.5
A duct shows mucinous metaplasia of lining cells with basally oriented nuclei and mucinous cytoplasm. The duct architecture is preserved, with an outer rim of myoepithelial cells, with florid usual ductal hyperplasia filling the lumen

Fig. 6.6
Mucinous metaplasia observed within an intraductal papilloma. The underlying papillary architecture is not well appreciated at this high magnification. Here, mucinous cells replace the lining epithelium of tubules within the intraductal papilloma. Some luminal mucin is also found in the glands lined by mucinous cells
Differential Diagnosis
Cystic Changes with Luminal Mucin Versus Mucocele-Like Lesion
The key difference between these lesions is the degree of distension of ducts by luminal mucin. Marked duct distension with mucin extravasation into surrounding stroma indicates a mucocele-like lesion. Calcifications superimposed on mucin may be observed in both conditions.
Mucinous Metaplasia Versus Mucoepidermoid Carcinoma
Mucoepidermoid carcinoma is a rare, salivary-gland type of breast cancer; in its low-grade variety, it may show a prevalence of mucous cells that can resemble mucinous metaplasia [3]. However, mucinous metaplasia does not show additional features of low-grade mucoepidermoid carcinoma such as the presence of intermediate and squamous cells, apart from the invasive nature of the latter (Fig. 6.7).


Fig. 6.7
Mucoepidermoid carcinoma. Invasive tumour islands incorporating mucinous cells (arrows) are seen within fibrotic stroma
Prognosis and Therapy Considerations
Breast cysts with luminal mucin and mucinous metaplasia have no clinical impact. Cysts can be accompanied by proliferative epithelial changes that may have corresponding risk implications for subsequent breast cancer development.
Mucocele-Like Lesions
Definition
Mucocele-like lesions (MLL) are defined by the presence of cysts and dilated ducts distended by mucin with associated rupture and mucin seepage into the surrounding breast stroma. The term “lesion” is preferred over “tumour”, as the accompanying epithelial changes are often not neoplastic. Associated epithelial alterations are assessed separately.
Clinical and Epidemiological Features
The incidence of MLL is not known, though these lesions are increasingly detected in the mammographic era because of the occurrence of calcifications. Large MLL may present clinically as breast lumps.
Imaging Features
Clustered, coarse microcalcifications with or without a lobulated mass may be observed (Fig. 6.8).


Fig. 6.8
Mucocele-like lesion. Magnification mammogram view shows a cluster of fine granular and amorphous microcalcifications together with a focus of coarse calcification (Courtesy of Dr. Lester Leong)
Pathologic Features
Macroscopic Pathology
Mucin-containing cysts within fibrous stroma and grittiness related to calcifications can be present (Figs. 6.9 and 6.10).



Fig. 6.9
Mucocele-like lesion with calcifications. Hookwire localisation excision specimen (hookwire has been removed) shows cysts with mucoid material on cut sections, within a fibrofatty parenchyma. Calcifications were identified histologically

Fig. 6.10
Mucocele-like lesion with ductal carcinoma in situ (DCIS). Macroscopically, viscid mucin is seen extruding from the cut surface. DCIS was found on histological examination
Microscopic Pathology
Cysts distended by luminal mucin with mucin extravasation into the stroma are present. The lining of the mucin-filled cysts may be flattened, attenuated benign epithelium, or it may show a variety of alterations including usual ductal hyperplasia, columnar cell change, columnar cell hyperplasia, flat epithelial atypia, atypical ductal hyperplasia (ADH), and ductal carcinoma in situ (DCIS). Coarse calcifications may be seen in luminal and extruded mucin (Figs. 6.11, 6.12, and 6.13).




Fig. 6.11
Benign mucocele-like lesion with calcifications. Cystically dilated ducts are lined by attenuated epithelium without any cytoarchitectural atypia. Calcifications are observed within the mucin

Fig. 6.12
Mucocele-like lesion with calcifications seen on core biopsy of mammographically detected calcifications

Fig. 6.13
Mucocele-like lesion on core biopsy. Cystically dilated ducts containing luminal mucin are present, with stromal mucin extravasation and scattered calcifications
Differential Diagnosis
MLL with ADH Versus MLL with Low Nuclear Grade DCIS
The distinction between MLL with ADH and MLL with low nuclear grade DCIS (Figs. 6.14 and 6.15) follows usual qualitative and quantitative criteria used to distinguish between these two lesions, with ADH diagnosed when duct spaces are only partially involved by the atypical epithelial population or when the lesional size does not exceed 2 mm. As MLL features dilated cysts, it may be difficult to apply the 2-mm size criterion. Using the involvement of two duct spaces as a diagnostic guide may be a practical alternative, although caution needs to be exercised in order not to overdiagnose small foci of atypical epithelial changes as DCIS. An appraisal of the entire lesion and a careful assessment of the extent of the atypical epithelial alterations are required.



Fig. 6.14
(a) Mucocele-like lesion with a borderline lesion for which the differential diagnosis is between atypical ductal hyperplasia versus low nuclear grade ductal carcinoma in situ. Excision biopsy shows a cluster of cystically dilated ducts filled with mucin, accompanied by coarse calcifications. Several ducts show architectural atypia with arches and micropapillary projections which may be difficult to appreciate at this magnification. (b) On higher magnification, the ducts show rigid arches and cribriform spaces, with involvement of at least two duct spaces. Luminal mucin and calcifications are present. The lesional extent measured more than 2 mm, fulfilling the size criterion of DCIS, although size assessment may be challenging in these markedly distended ducts. Lesser degrees of involvement warrant a diagnosis of atypical ductal hyperplasia (ADH)

Fig. 6.15




Mucocele-like lesion with ductal carcinoma in situ. (a) Hookwire localisation excision biopsy of radiologically detected calcifications in the right breast shows a cluster of mucin-filled cysts near the tissue edge (black arrow). A yellowish-brown nodule (white arrow) is present, representing the previous mammotome biopsy site. Histological findings of the prior mammotome biopsy showed a mucocele-like lesion with atypical ductal hyperplasia accompanied by calcifications. (b) Corresponding histological section reveals distended cysts (black arrow) aggregated near the inked surgical edge of the tissue. The previous mammotome biopsy site is noted (white arrow). (c) The cystically dilated ducts are lined by flattened epithelium that is punctuated by rigid epithelial arches. Mucin distends the duct lumens, with spillage into the surrounding stroma. While the degree of epithelial architectural atypia depicted in this illustration may not qualify for a diagnosis of low grade ductal carcinoma in situ and may be considered atypical ductal hyperplasia in the absence of further ductal epithelial abnormalities, presence of a greater extent (> 2mm; or 2 or more affected ducts) of cytoarchitecturally abnormal epithelial changes could be regarded as ductal carcinoma in situ. Careful assessment of the extent of involvement is important to avoid overdiagnosing small foci of atypical epithelial alterations as ductal carcinoma in situ. (d) Higher magnification shows cytoarchitecturally abnormal epithelial changes in the wall of this distended duct. Stiff epithelial arches with secondary rigid lumens are seen. These abnormal alterations were found in several contiguous sections of the breast tissue, indicating a significant disease extent fulfilling the size criterion of low nuclear grade ductal carcinoma in situ
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


