Figure 8-1. Mania-minded treatments. Although the ideal “mood stabilizer” would treat both mania and bipolar depression while also preventing episodes of either pole, in reality there is as yet no evidence to suggest that any single agent can achieve this consistently. Rather, different agents may be efficacious for different phases of bipolar disorder. As shown here, some agents seem to be “mania-minded” and thus able to “treat from above” and/or “stabilize from above” – in other words, to reduce and/or prevent symptoms of mania.
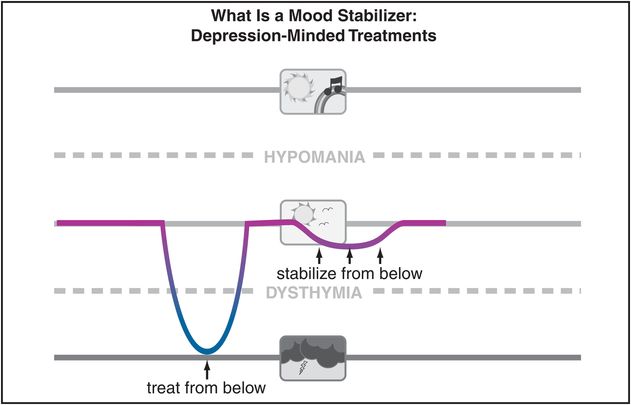
Figure 8-2. Depression-minded treatments. Although the ideal “mood stabilizer” would treat both mania and bipolar depression while also preventing episodes of either pole, as mentioned for Figure 8-1, in reality there is as yet no evidence to suggest that any single agent can achieve this consistently. Rather, different agents may be efficacious for different phases of bipolar disorder. As shown here, some agents seem to be “depression-minded” and thus able to “treat from below” and/or “stabilize from below” – in other words, to reduce and/or prevent symptoms of bipolar depression.
Lithium, the classic mood stabilizer
Bipolar disorder has classically been treated with lithium for more than 50 years. Lithium is an ion whose mechanism of action is not certain. Candidates for its mechanism of action are various signal transduction sites beyond neurotransmitter receptors (Figure 8-3). This includes second messengers, such as the phosphatidyl inositol system, where lithium inhibits the enzyme inositol monophosphatase; modulation of G proteins; and most recently, regulation of gene expression for growth factors and neuronal plasticity by interaction with downstream signal transduction cascades, including inhibition of GSK-3 (glycogen synthase kinase 3) and protein kinase C (Figure 8-3).
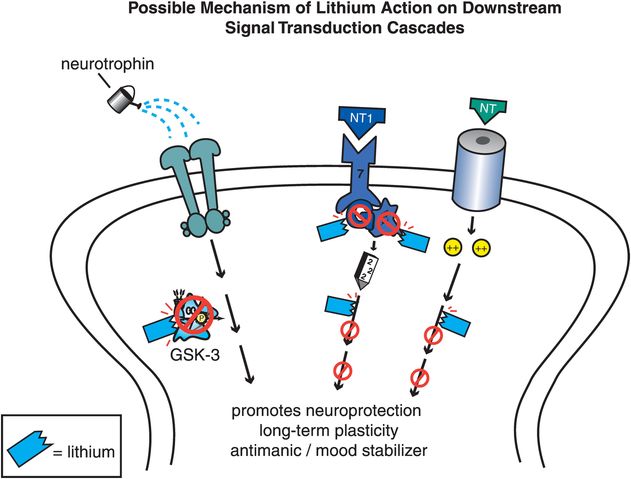
Figure 8-3. Lithium’s mechanism of action. Although lithium is the oldest treatment for bipolar disorder, its mechanism of action is still not well understood. Several possible mechanisms exist and are shown here. Lithium may work by affecting signal transduction, perhaps through its inhibition of second-messenger enzymes such as inositol monophosphatase (right), by modulation of G proteins (middle), or by interaction at various sites within downstream signal transduction cascades (left).
However lithium works, it is proven effective in manic episodes and in maintenance of recurrence, especially for manic episodes and perhaps to a lesser extent for depressive episodes. Lithium is well established to help prevent suicide in patients with mood disorders. It is also used to treat depressive episodes in bipolar disorder as an augmenting agent to antidepressants for unipolar depression, as mentioned in Chapter 7, but is not formally approved for these uses. A number of factors have led to an unfortunate decline in the use of lithium in recent years, including the entry of multiple new treatment options into the therapeutic armamentarium for bipolar disorder, the side effects of lithium, and the monitoring burden that is part of prescribing lithium. The modern use of lithium by experts departs from its classic use as a high-dose monotherapy for euphoric mania, with lithium utilized now as one member of a portfolio of treatments, often allowing once-a-day administration and lower doses when combined with other mood stabilizers. Lithium has equal or better efficacy in bipolar disorder compared to valproate for manic, depressive, or mixed episodes, although valproate is often more frequently prescribed. Anticonvulsants including valproate have been controversially and not completely convincingly linked to causing suicidality, whereas lithium actually reduces suicide in patients with bipolar disorder. In fact, some provocative studies from Austria to Texas to Japan suggest that the more lithium mobilized by rain from rocks and soil and then dissolved in drinking water, the lower the suicide rate in the general population as well! Another potential use of lithium arises from the notion that inhibition of GSK-3 by lithium could theoretically inhibit the phosphorylation of tau (τ) proteins and thus slow the formation of plaques and tangles in Alzheimer’s disease. A few studies have suggested that lithium can prevent progression from mild cognitive impairment to Alzheimer’s disease and reduce phosphorylated τ levels, especially if given for a long period of time (> 1 year), and even at low doses. This remains controversial and needs replication in larger studies, but is certainly an interesting development to monitor.
Well-known side effects of lithium include gastrointestinal symptoms such as dyspepsia, nausea, vomiting, and diarrhea, as well as weight gain, hair loss, acne, tremor, sedation, decreased cognition, and incoordination. There are also long-term adverse effects upon the thyroid and kidney. Lithium has a narrow therapeutic window, requiring monitoring of plasma drug levels. Modern use of lithium often includes dosing at the lower end of the therapeutic window and combining lithium with other mood stabilizers.
Anticonvulsants as mood stabilizers
Based upon theories that mania may “kindle” further episodes of mania, a logical parallel with seizure disorders was drawn, since seizures can “kindle” more seizures. Thus, several anticonvulsants are used to treat bipolar disorder, some with better evidence of efficacy than others (Table 8-1). Since the first anticonvulsants tested, namely carbamazepine and valproate, proved effective in treating the manic phase of bipolar disorder, this has led to the idea than any anticonvulsant would be a mood stabilizer, especially for mania. However, this has not proven to be the case, as anticonvulsants do not all act by the same pharmacological mechanisms. Numerous anticonvulsants are discussed below, including not only those with proven efficacy in different phases of bipolar disorder but also those with dubious efficacy in bipolar disorder (Table 8-1).
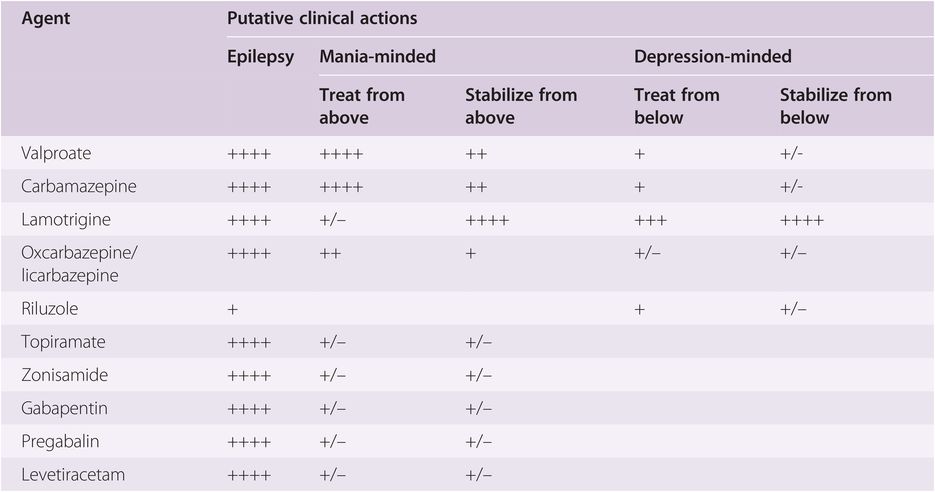
Anticonvulsants with proven efficacy in bipolar disorder
Valproic acid
As for all anticonvulsants, the exact mechanism of action of valproic acid (also, valproate sodium, or valproate) is uncertain; however, even less may be known about the mechanism of valproate than for other anticonvulsants. Various hypotheses are discussed here, and summarized in Figures 8-4 though 8-7. At least three possibilities exist for how valproic acid works: inhibiting voltage-sensitive sodium channels (Figure 8-5), boosting the actions of the neurotransmitter GABA (γ-aminobutyric acid) (Figure 8-6), and regulating downstream signal transduction cascades (Figure 8-7). It is not known whether these actions explain the mood-stabilizing actions, the anticonvulsant actions, the anti-migraine actions, or the side effects of valproic acid. Obviously, this simple molecule has multiple and complex clinical effects, and research is trying to determine which of the various possibilities explain the mood-stabilizing effects of valproic acid so that new agents with more efficacy and fewer side effects can be developed by targeting the relevant pharmacological mechanism for bipolar disorder.
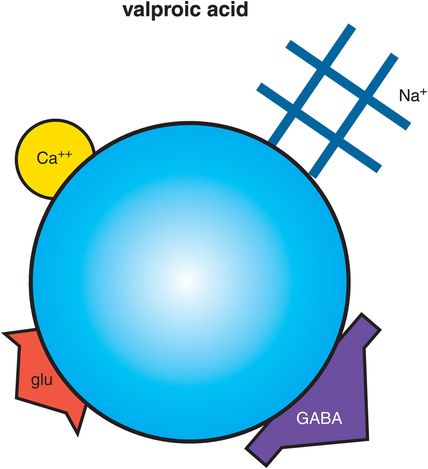
Figure 8-4. Valproic acid. Shown here is an icon of the pharmacological actions of valproic acid, an anticonvulsant used in the treatment of bipolar disorder. Valproic acid (also valproate) may work by interfering with voltage-sensitive sodium channels (VSSCs), enhancing the inhibitory actions of γ-aminobutyric acid (GABA), and regulating downstream signal transduction cascades, although which of these actions may be related to mood stabilization is not clear. Valproate may also interact with other ion channels, such as voltage-sensitive calcium channels (VSCCs), and also indirectly block glutamate actions.
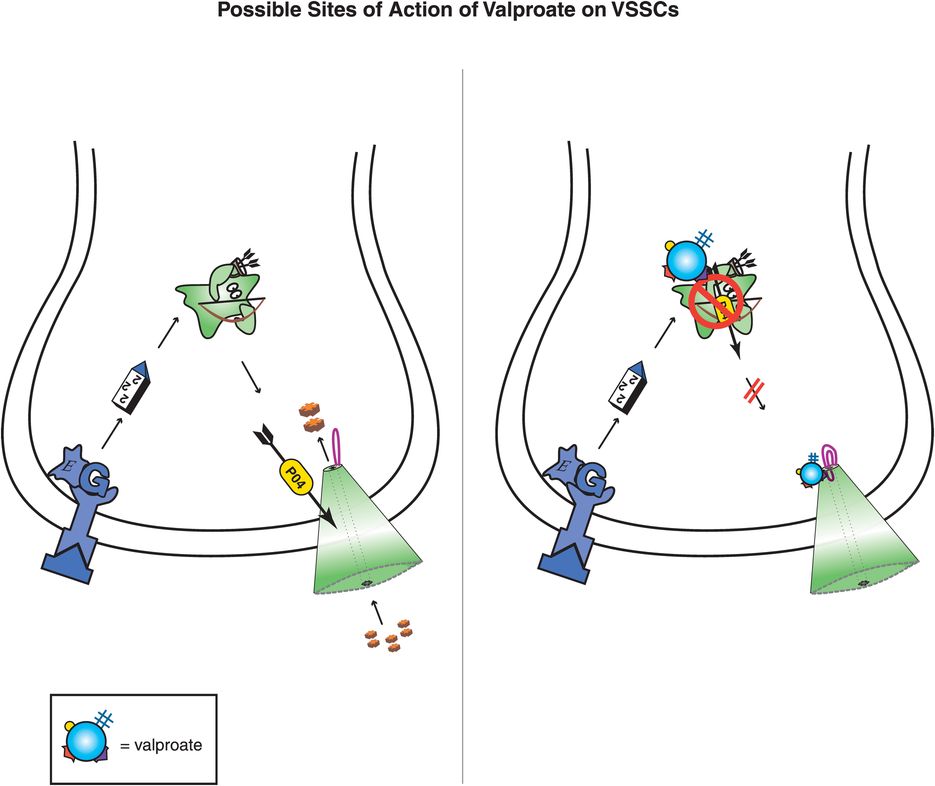
Figure 8-5. Possible sites of action of valproate on voltage-sensitive sodium channels (VSSCs). Valproate may exert antimanic effects by changing the sensitivity of VSSCs, perhaps by directly binding to channel subunits or inhibiting phosphorylating enzymes that regulate the sensitivity of these ion channels. Inhibition of VSSCs would lead to reduced sodium influx and, in turn, potentially to reduced glutamate excitatory neurotransmission, which is a possible mechanism for mania efficacy.
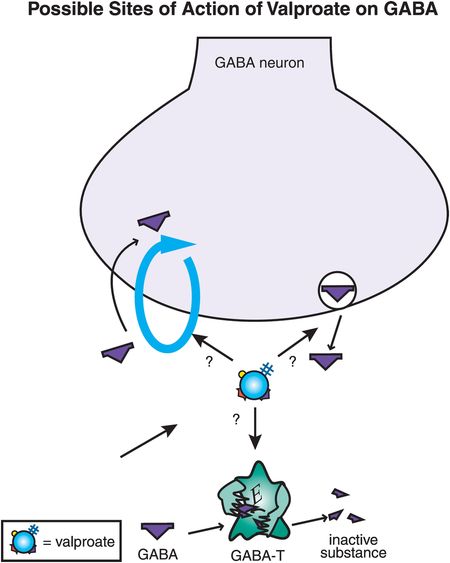
Figure 8-6. Possible sites of action of valproate on γ-aminobutyric acid (GABA). Valproate’s antimanic effects may be due to enhancement of GABA neurotransmission, perhaps by inhibiting GABA reuptake, enhancing GABA release, or interfering with the metabolism of GABA by GABA transaminase (GABA-T).
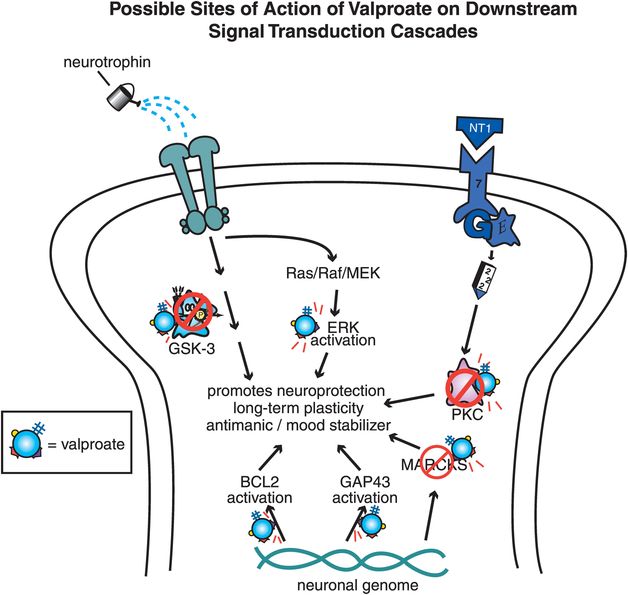
Figure 8-7. Possible sites of action of valproate on downstream signal transduction cascades. Valproate has been shown to have multiple downstream effects on signal transduction cascades, which may be involved in its antimanic effects. Valproate inhibits glycogen synthase kinase 3 (GSK-3), protein kinase C (PKC), and myristolated alanine-rich C kinase substrate (MARCKS). In addition, valproate activates signals that promote neuroprotection and long-term plasticity, such as extracellular signal-regulated kinase (ERK), cytoprotective protein B-cell lymphoma/leukemia-2 gene (BCL2), and GAP43.
One hypothesis to explain mood-stabilizing antimanic actions is the possibility that valproate acts to diminish excessive neurotransmission by diminishing the flow of ions through voltage-sensitive sodium channels (VSSCs) (Figure 8-5). VSSCs are discussed in Chapter 3 and illustrated in Figures 3-19 through 3-21. No specific molecular site of action for valproate has been clarified, but it is possible that valproate may change the sensitivity of sodium channels by altering their phosphorylation, either by binding directly to the VSSC or its regulatory units or by inhibiting phosphorylating enzymes (Figure 8-5). If less sodium is able to pass into neurons, this may lead to diminished release of glutamate and therefore less excitatory neurotransmission, but this is only a theory. There may be additional effects of valproate on other voltage-sensitive ion channels, but these are poorly characterized and may relate to side effects as well as to therapeutic effects.
Another idea is that valproate enhances the actions of GABA, by increasing its release, decreasing its reuptake, or slowing its metabolic inactivation (Figure 8-6). The direct site of action of valproate that causes the enhancement of GABA remains unknown, but there is good evidence that the downstream effects of valproate ultimately do result in more GABA activity, and thus more inhibitory neurotransmission, possibly explaining antimanic actions.
Finally, a number of downstream actions on complex signal transduction cascades have been described in recent years (Figure 8-7). Like lithium, valproate may inhibit GSK-3, but it may also target many other downstream sites, from blockade of phosphokinase C (PKC) and MARCKS (myristolated alanine-rich C kinase substrate), to activating various signals that promote neuroprotection and long-term plasticity such as ERK kinase (extracellular signal-regulated kinase), BCL2 (cytoprotective protein B-cell lymphoma/leukemia-2 gene), GAP43, and others (Figure 8-7). The effects of these signal transduction cascades are only now being clarified, and which of these possible effects of valproate might be relevant to mood-stabilizing actions is not yet understood.
Valproate is proven effective for the acute manic phase of bipolar disorder and is commonly used long term to prevent recurrence of mania, although its prophylactic effects have not been as well established as its acute effects in mania. Antidepressant actions of valproate have also not been well established, nor has it been shown to convincingly stabilize against recurrent depressive episodes, but there may be some efficacy for the depressed phase of bipolar disorder in some patients. Some experts believe valproic acid is more effective than lithium for rapid cycling and mixed episodes of mania. In reality, such episodes are very difficult to treat, and combinations of two or more mood stabilizers, including lithium plus valproate, are usually in order. As mentioned for lithium, valproate can also be utilized once a day in doses that are towards the bottom of the therapeutic range in combination with other mood stabilizers such as lithium to improve tolerability and compliance. For optimum efficacy, it may be ideal to push the dose of valproate, but no drug works if your patient refuses to take it, and valproic acid often has unacceptable side effects such as hair loss, weight gain, and sedation. Certain problems can be avoided by lowering the dose, but this will generally lower efficacy, and thus there may be the requirement to combine it with other mood stabilizers when valproate is given in lower doses. Some side effects may be related more to chronicity of exposure rather than to dose and thus may not be avoided by reducing the dose. This includes warnings for liver and pancreatic effects, fetal toxicities such as neural tube defects, concerns about weight gain and metabolic complications, and possible risk of amenorrhea and polycystic ovaries in women of childbearing potential. A syndrome of menstrual disturbances, polycystic ovaries, hyperandrogenism, obesity, and insulin resistance may be associated with valproic acid therapy in such women.
Carbamazepine
Carbamazepine (Figure 8-8) was actually the first anticonvulsant to be shown effective in the manic phase of bipolar disorder, but it did not receive formal FDA approval until recently as a once-daily controlled-release formulation. Although carbamazepine and valproate both act effectively on the manic phase of bipolar disorder (Table 8-1), they appear to have different pharmacological mechanisms of action, including different side-effect profiles. Thus, carbamazepine is hypothesized to act by blocking voltage-sensitive sodium channels (VSSCs) (Figure 8-8), perhaps at a site within the channel itself, also known as the α subunit of VSSCs (Figure 8-9). As mentioned earlier, VSSCs are discussed in Chapter 3 and illustrated in Figures 3-19 through 3-21. The hypothesized action of carbamazepine upon the α subunit of VSSCs (Figure 8-9) is different from the hypothesized actions of valproate on these sodium channels (Figure 8-5), but may be similar to how the anticonvulsants oxcarbazepine and its active metabolite eslicarbazepine also act.
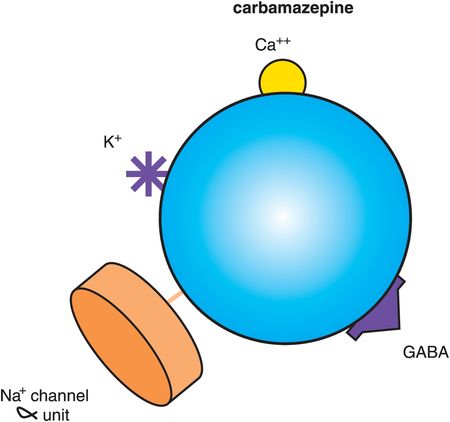
Figure 8-8. Carbamazepine. Shown here is an icon of the pharmacological actions of carbamazepine, an anticonvulsant used in the treatment of bipolar disorder. Carbamazepine may work by binding to the α subunit of voltage-sensitive sodium channels (VSSCs) and could perhaps have actions at other ion channels for calcium and potassium. By interfering with voltage-sensitive channels, carbamazepine may enhance the inhibitory actions of γ-aminobutyric acid (GABA).
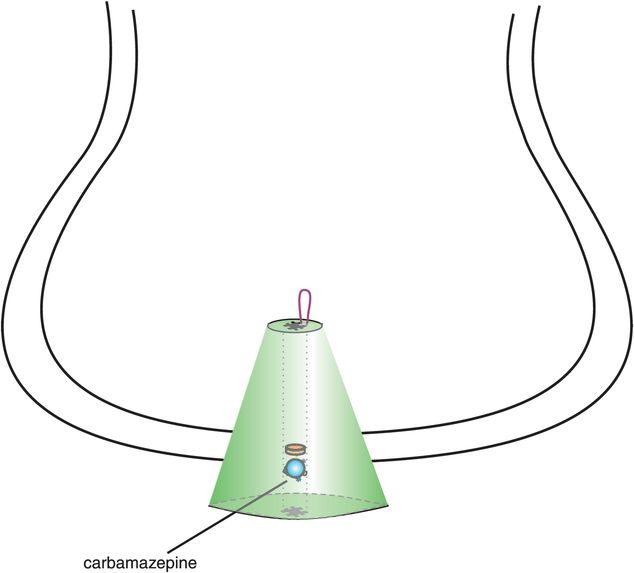
Figure 8-9. Binding site of carbamazepine. Carbamazepine is believed to bind to a site located within the open channel conformation of the voltage-sensitive sodium channel (VSSC) α subunit.
Although both carbamazepine and valproate are anticonvulsants and treat mania from above, there are many differences between these two anticonvulsants. For example, valproate is proven effective in migraine, but carbamazepine is proven effective in neuropathic pain. Furthermore, carbamazepine has a different side-effect profile than valproate, including suppressant effects upon the bone marrow, requiring initial monitoring of blood counts, and notable induction of the cytochrome P450 (CYP) enzyme 3A4. Carbamazepine is sedating and can cause fetal toxicity such as neural tube defects.
Lamotrigine
Lamotrigine (Figure 8-10) is approved as a mood stabilizer to prevent recurrence of both mania and depression. There are many curious things about lamotrigine as a mood stabilizer. First, the FDA has not approved its use for bipolar depression, yet most experts believe that lamotrigine is effective for bipolar depression. In fact, given the growing concern about antidepressants inducing mania, causing mood instability, and increasing suicidality in bipolar disorder, lamotrigine has largely replaced antidepressants as a first-line recommendation in most treatment guidelines for bipolar depression. In that regard, lamotrigine has transformed the treatment of this difficult phase of bipolar disorder as one of the very few agents that seem to be effective for bipolar depression based upon results seen in clinical practice rather than from evidence derived from clinical trials.
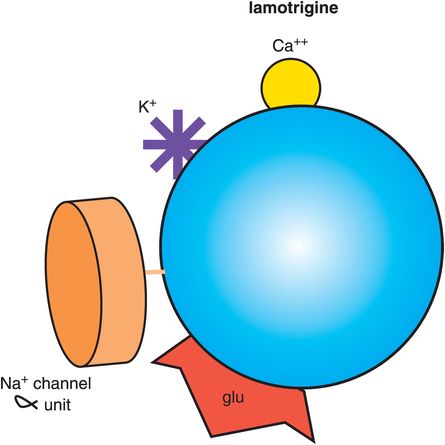
Figure 8-10. Lamotrigine. Shown here is an icon of the pharmacological actions of lamotrigine, an anticonvulsant used in the treatment of bipolar disorder. Lamotrigine may work by blocking the α subunit of voltage-sensitive sodium channels (VSSCs) and could perhaps also have actions at other ion channels for calcium and potassium. Lamotrigine is also thought to reduce the release of the excitatory neurotransmitter glutamate.
A second interesting thing about lamotrigine is that even though it has some overlapping mechanistic actions with carbamazepine, namely binding to the open channel conformation of VSSCs (Figures 8-9 and 8-11), lamotrigine is not approved for bipolar mania. Perhaps its actions are not potent enough at sodium channels, or perhaps the long titration period required when starting this drug makes it difficult to show any useful effectiveness for mania, which generally requires treatment with drugs that can work quickly.
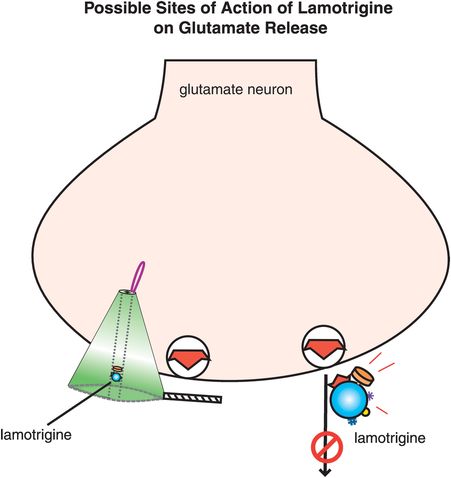
Figure 8-11. Possible site of action of lamotrigine on glutamate release. It is possible that lamotrigine reduces glutamate release through its blockade of voltage-sensitive sodium channels (VSSCs). Alternatively, lamotrigine may have this effect via an additional synaptic action that has not yet been identified.
A third aspect of lamotrigine that is unusual for an antidepressant mood stabilizer is its tolerability profile. Lamotrigine is generally well tolerated for an anticonvulsant, except for its propensity to cause rashes, including (rarely) the life-threatening Stevens–Johnson syndrome (toxic epidermal necrolysis). Rashes caused by lamotrigine can be minimized by very slow up-titration of drug during initiation of therapy, avoiding or managing drug interactions such as those with valproate that raise lamotrigine levels, and by understanding how to identify and manage serious rashes, including being able to distinguish them from benign rashes (see discussion of lamotrigine in Stahl’s Essential Psychopharmacology: the Prescriber’s Guide).
Finally, lamotrigine seems to have some unique aspects to its mechanism of action (Figure 8-11), namely to reduce the release of the excitatory neurotransmitter glutamate. It is not clear whether this action is secondary to blocking the activation of VSSCs (Figure 8-11) or to some additional synaptic action. Reducing excitatory glutamatergic neurotransmission, especially if excessive during bipolar depression, may be a unique action of lamotrigine and explain why it has such a different clinical profile as a treatment from below and a stabilizer from below for bipolar depression.
Anticonvulsants with uncertain or doubtful efficacy in bipolar disorder
Oxcarbazepine/eslicarbazepine
Oxcarbazepine is structurally related to carbamazepine, but is not a metabolite of carbamazepine. Oxcarbazepine is actually not the active form of the drug, but a prodrug that is immediately converted into a 10-hydroxy derivative, also called the monohydroxy derivative, that most recently has been named licarbazepine. The active form of licarbazepine is the S enantiomer, known as eslicarbazepine. Thus, oxcarbazepine really works via conversion to eslicarbazepine.
Oxcarbazepine is well known as an anticonvulsant with a presumed mechanism of anticonvulsant action the same as that for carbamazepine, namely, binding to the open channel conformation of the VSSC at a site within the channel itself on the α subunit (as in Figure 8-9). However, oxcarbazepine seems to have some important differences from carbamazepine, including being less sedating, having less bone-marrow toxicity, and having fewer CYP 3A4 interactions, making it a more tolerable agent that is easier to dose. On the other hand, oxcarbazepine has never been proven to work as a mood stabilizer. Nevertheless, because of a similar postulated mechanism of action but a better tolerability profile, oxcarbazepine has been utilized “off-label” by many clinicians, especially for the manic phase of bipolar disorder. There is now active investigation of the active moiety eslicarbazepine as a potential mood stabilizer.
Topiramate
Topiramate is another compound approved as an anticonvulsant and for migraine, and recently in combination with bupropion for weight loss in obesity. Topiramate has been tested in bipolar disorder, but with ambiguous results (Table 8-1). It does seem to be associated with weight loss and is sometimes given as an adjunct to mood stabilizers that cause weight gain, but can cause unacceptable sedation in some patients. Topiramate is also being tested in various substance-abuse disorders, including stimulant abuse and alcoholism. However, topiramate is not clearly effective as a mood stabilizer, neither from evidence-based randomized controlled trials (which are not consistently positive) nor from clinical practice.
The reason that topiramate may not have the robust efficacy of valproate or carbamazepine in the manic phase, nor of lamotrigine in the depressed and maintenance phases of bipolar disorder, is that it has a different mechanism of action from any of these agents. The exact binding site for topiramate is not known (see Figure 10-19), but it seems to enhance GABA function and reduce glutamate function by interfering with both sodium and calcium channels, but in a different way and at a different site than the previously discussed anticonvulsants. In addition, topiramate is a weak inhibitor of carbonic anhydrase. Topiramate is now considered an adjunctive treatment for bipolar disorder, perhaps helpful for weight gain, insomnia or anxiety, or possibly for comorbid substance abuse, but not necessarily as a mood stabilizer per se. It is also under investigation, in combination with phentermine, as a treatment for weight loss in obesity, which will be discussed in Chapter 14.
Gabapentin and pregabalin
These anticonvulsants seem to have little or no action as mood stabilizers, yet are robust treatments for various pain conditions, from neuropathic pain to fibromyalgia, and for various anxiety disorders; they are discussed in more detail in Chapters 9 and 10, dealing with anxiety and pain. Gabapentin and pregabalin are now classified as α2δ ligands, since they are known to bind selectively and with high affinity to the α2δ site of voltage-sensitive calcium channels (VSCCs) (see discussion in Chapter 10, and Figures 10-14 through 10-18). It appears that blocking these VSCCs when they are open and in use causes improvement of seizures, pain, and anxiety but not stabilization of mood. That is, “use-dependent” blockade of VSCCs prevents the release of neurotransmitters such as glutamate in pain pathways and anxiety pathways and also prevents seizures, but does not appear to affect the mechanism involved in bipolar disorder, since clinical trials of these agents in bipolar disorder show unconvincing mood stabilization. However, many bipolar patients do experience chronic pain, anxiety, and insomnia, and gabapentin and pregabalin may be useful adjunctive treatments to effective mood stabilizers, even though they do not appear to be robustly effective as mood stabilizers themselves. This is not surprising given the very different mechanism of action of these compounds as selective α2δ ligands on calcium channels (Figures 10-14 through 10-18), compared to the mechanisms of proven mood stabilizers such as valproate, carbamazepine, and lamotrigine on sodium channels (discussed above).
Calcium channel blockers (L-type)
There are several types of calcium channels, not only the N or P/Q channels linked to secretion of neurotransmitters, targeted by α2δ ligands, and discussed in Chapter 3 (see Figures 3-23 and 3-24) but also L channels localized on vascular smooth muscle that are targeted by various antihypertensive and antiarrhythmic drugs commonly called “calcium channel blockers.” L-type channels are located on neurons where their function is still being debated, and some anecdotal evidence suggests that calcium channel blockers, especially dihydropyridine-type calcium channel blockers, may be useful for some patients with bipolar disorder.
Riluzole
This agent has anticonvulsant actions in preclinical models, but was developed to slow the progression of amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease). Theoretically, riluzole binds to VSSCs and prevents glutamate release in an action similar to that postulated for lamotrigine (Figure 8-11). The idea is that diminishing glutamate release in ALS would prevent the postulated excitotoxicity that may be causing death of motor neurons in ALS. Excessive glutamate activity may be occurring not only in ALS, but may also occur in bipolar depression, although not necessarily so severely as to cause widespread neuronal loss.
Due to riluzole’s putative action on preventing glutamate release, it has been tested in case series in a number of treatment-resistant conditions hypothetically linked to excessive glutamate activity, including not only bipolar depression but also treatment-resistant unipolar depression and anxiety disorders, with some promising initial results. There is great need for another agent that has the same clinical effects as lamotrigine. The problem with riluzole is that it is quite expensive and has frequent liver-function abnormalities associated with its use.
Atypical antipsychotics as mood stabilizers: not just for psychotic mania
When atypical antipsychotics were approved for schizophrenia, it was not surprising that these agents would work for psychotic symptoms associated with mania, since the D2 antagonist actions predict efficacy for psychosis in general (discussed in Chapter 5). However, it was somewhat surprising when these agents proved effective for the core nonpsychotic symptoms of mania and for maintenance treatment to prevent the recurrence of mania. These latter actions are similar to lithium and various anticonvulsants that act by very different mechanisms. More surprising yet is that some atypical antipsychotics are effective for bipolar depression. The question that arises is, how do atypical antipsychotics work as mood stabilizers? Also, do they act as mood stabilizers by the same pharmacologic mechanism as they do as antipsychotics? Finally, do they work for the symptoms of mania by the same pharmacologic mechanisms as they do for bipolar depression?
Putative pharmacologic mechanism of atypical antipsychotics in mania and bipolar depression
The answer to the question of how atypical antipsychotics work in mania is that we do not really know (Figure 5-36). In fact, theories about atypical antipsychotic pharmacologic actions in bipolar disorder are less well developed than they are for schizophrenia, such as those discussed extensively in Chapter 5. Indeed, it is still a quandary how bipolar disorder itself can create seemingly opposite symptoms during various phases of the illness, as well as the combination of both manic and depressive symptoms simultaneously. Ideas about dysfunctional circuits in the depressed phase of bipolar disorder (discussed in Chapter 6 and illustrated in Figure 6-45) are contrasted with different dysfunctions in both overlapping and distinctive circuits during the manic phase of the illness (discussed in Chapter 6 and illustrated in Figure 6-48). Rather than being conceptualized as having activity that is simply “too low” in depression and “too high” in mania, the idea is that dysfunctional circuits in bipolar disorder are “out of tune” and chaotic. According to this notion, mood stabilizers have the ability to “tune” dysfunctional circuits, increasing the efficiency of information processing in symptomatic circuits, thus decreasing symptoms whether manic or depressed.
If so, the D2 antagonist or partial agonist properties of atypical antipsychotics as well as conventional antipsychotics may account for reduction of psychotic symptoms in mania, but the 5HT2A antagonist and 5HT1A partial agonist properties of atypical antipsychotics may account for reduction of nonpsychotic manic and depressive symptoms by some (but not all) atypical antipsychotics. This could occur via reduction of glutamate hyperactivity from overly active pyramidal neurons by 5HT2A antagonist actions (discussed in Chapter 5 and illustrated in Figure 5-15). This could reduce symptoms associated with glutamate hyperactivity, which could include both manic and depressive symptoms, depending upon the circuit involved. Anti-glutamate actions of atypical antipsychotics are consistent with the known pharmacologic mechanisms of several known anticonvulsants that are also mood stabilizers. Adding together different mechanisms that decrease excessive glutamate activity could explain the observed therapeutic benefits of combining atypical antipsychotics with proven anticonvulsant mood stabilizers.
Several other mechanisms are feasible explanations for how certain atypical antipsychotics work to improve symptoms in the depressed phase of bipolar disorder (discussed in Chapter 5 and illustrated in Figures 5-36, 5-37, 5-52, 5-60, 5-61). Thus, numerous mechanisms of different atypical antipsychotics can increase the availability of monoamine neurotransmitters serotonin, dopamine, and norepinephrine, known to be critical in the action of antidepressants in unipolar depression. There are very different pharmacologic properties of one atypical antipsychotic compared to another, and this could potentially explain not only why some atypical antipsychotics have different actions than others in bipolar disorder, but also why some bipolar patients respond to one atypical antipsychotic and not another. Thus, all atypical antipsychotics are approved for schizophrenia, and most are approved for mania, but only one for bipolar depression (quetiapine), with another one having multiple positive clinical trials in bipolar depression (lurasidone). Although these differences in mood-stabilizer approvals for individual atypical antipsychotics may be somewhat of an artifact of commercial considerations and lack of completion of clinical trials for some of the newer agents, it may also reflect differing portfolios of pharmacologic actions among those properties that might have antidepressant actions (Figure 5-36). Much further research must be completed before we will know the reason why atypical antipsychotics may work in mania or in bipolar depression. In the meantime, these agents as a class provide some of the broadest efficacy in bipolar disorder available, indeed broader than for most anticonvulsants and comparable or better than that for lithium. Increasingly, therefore, the treatment of bipolar disorder is not only with two or more agents, but with one of those agents being an atypical antipsychotic.
Other agents used in bipolar disorder
Benzodiazepines
Although benzodiazepines are not formally approved as mood stabilizers, they nevertheless provide valuable adjunctive treatment to proven mood stabilizers, especially in emergent situations. Intramuscular or oral administration of benzodiazepines can have a calming action immediately, and provide valuable time for mood stabilizers with a longer onset of action to begin working. Also, benzodiazepines are quite valuable for patients on an as-needed basis for intermittent agitation, insomnia and incipient manic symptoms. Skilled intermittent use can leverage the mood-stabilizing actions of concomitant mood stabilizers and prevent eruption of more severe symptoms and possibly avoid rehospitalization. Of course, benzodiazepines should be administered with caution, especially acutely to patients with comorbid substance abuse, or chronically to any patient. The mechanism of action of benzodiazepines on GABAA receptors is discussed in further detail in Chapter 9.
Modafinil and armodafinil
The wake-promoting agents modafinil and the active enantiomer armodafinil have both been tested in bipolar depression with positive results. Large multicenter trials of armodafinil as adjunctive treatment to atypical antipsychotics in bipolar depression are promising. These agents, sometimes classified as stimulants but known to be blockers of the dopamine transporter (DAT), are discussed in greater detail in Chapter 11.
Hormones and natural products
The omega-3 fatty acids EPA (eicosapentanoic acid) and DHA (docosohexanoic acid) have been proposed as mood stabilizers, or as natural products that may boost the actions of proven mood stabilizers with few if any side effects. EPA is an essential fatty acid and can be metabolized to DHA, and is a normal component of a diet that contains fish. Both EPA and DHA are found in large quantity in the brain, especially in cell membranes. Recent investigations suggest that omega-3 fatty acids may inhibit PKC (protein kinase C), not unlike the actions described earlier for valproate and illustrated in Figure 8-7. Studies of omega-3 fatty acids are ongoing and suggestive, but they have not yet been proven effective in bipolar disorder.
Inositol is a natural product linked to second-messenger systems and signal transduction cascades, especially for the phosphatidyl inositol signals related to various neurotransmitter receptors such as the 5HT2A receptor. Inositol has been studied in bipolar disorder and in treatment-resistant bipolar depression, where it may be as effective as an augmenting agent to antidepressants as approved mood stabilizers such as lamotrigine and risperidone. Further studies of inositol are necessary.
The centrally active form of the vitamin folate, L-methylfolate, is discussed extensively in Chapter 7 and illustrated in Figures 7-71 through 7-74. L-Methylfolate could theoretically boost monoamine neurotransmitter function in bipolar depression, but has not been widely studied in controlled trials. An additional rationale for utilizing L-methylfolate in bipolar disorder is because several anticonvulsants interfere with folate absorption or folate metabolism. Thus, bipolar patients who are partial responders to mood-stabilizing anticonvulsants (especially lamotrigine, valproate, and carbamazepine, but perhaps other anticonvulsants as well), or who lose their response, may be considered candidates for taking L-methylfolate.
Some investigators note that thyroid hormone, especially T3, may stabilize mood in some patients with bipolar disorder. This is not well researched and is somewhat controversial, especially for long-term use.
Antidepressants: do they make you bipolar?
Increasingly, it seems that antidepressants either do not work, or may worsen the situation for some patients who have bipolar disorder, causing destabilization of mood with induction of mania or hypomania, rapid cycling or mixed states or even suicidality. There is even an ongoing debate about whether antidepressants can cause someone to develop bipolar disorder who does not have this condition prior to taking an antidepressant, proposing that bipolar disorder could even be a complication of antidepressant treatment. Although this possibility is still under investigation, there is now little debate about the possibility that antidepressants, perhaps especially tricyclic antidepressants, can activate bipolar disorder in patients known to have a bipolar spectrum disorder.
Based upon current evidence, it seems likely that someone who develops bipolar disorder after taking an antidepressant is an individual who already has bipolar disorder, but the condition may have been previously undiagnosed, wrongly diagnosed, or “unmasked,” but not caused by antidepressant treatment. This is a particularly problematic issue for young patients, who may present with unipolar depressive symptoms before they express any manic or hypomanic symptoms, and who may be particularly vulnerable therefore both to misdiagnosis and to antidepressant-induced activation and suicidality.
So how do you know to whom you can give an antidepressant? Recommendations for use of antidepressants in patients with known bipolar disorder, who are at risk for bipolar disorder, or who have had activation of mania on antidepressants are still evolving. Currently, use of antidepressants for individuals in these situations must be considered on a case-by-case basis. Most experts agree that antidepressant monotherapy is generally to be avoided in such individuals, and that treatment of depression in bipolar disorder should start with other options such as lamotrigine, lithium, and/or atypical antipsychotics as monotherapies or in combination (Figure 8-12). Whether one can add an antidepressant to these agents in patients with bipolar depression who do not have robust treatment responses to these first-line agents is the subject of current debate. Many treatment guidelines do provide for use of antidepressants in combination with mood stabilizers, perhaps preferring bupropion the most and tricyclic antidepressants the least, but when to do this remains controversial, dependent somewhat on the results of ongoing studies, and upon where in the world you practice and were trained. Thus, common sense, integration of one’s clinical experience, and keeping up with this evolving area of psychopharmacology is now considered the best practice.
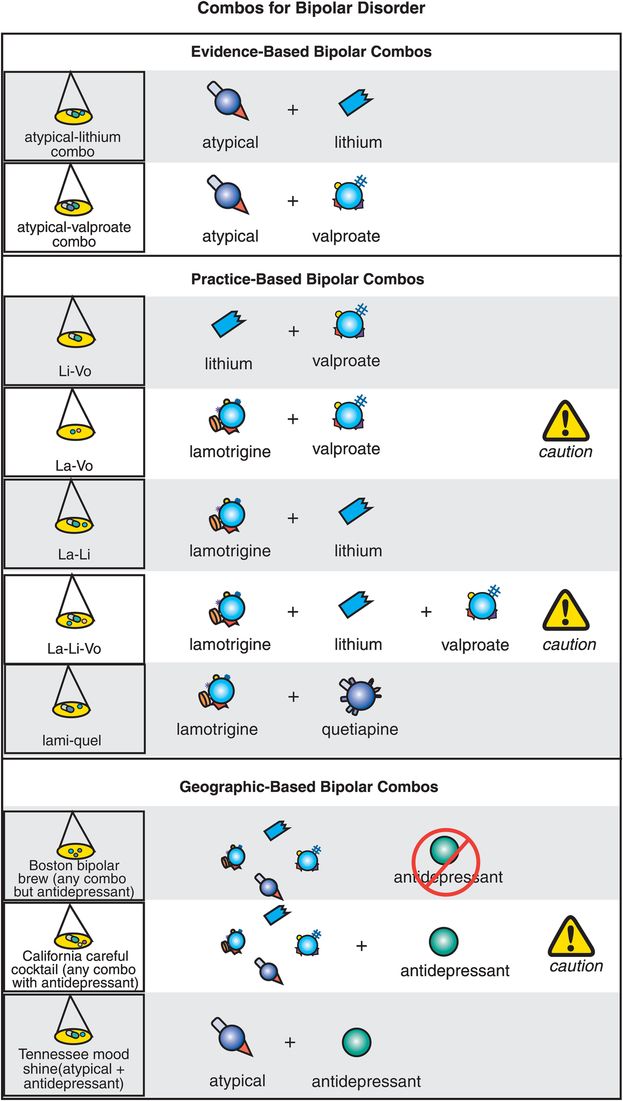
Figure 8-12. Bipolar disorder combinations. Most patients with bipolar disorder will require treatment with two or more agents. The combinations with the most evidence include addition of an atypical antipsychotic to either lithium (atypical–lithium combo) or valproate (atypical–valproate combo). Combinations that are not well studied in controlled trials but that have some practice-based evidence include lithium plus valproate (li-vo), cautious use of lamotrigine plus valproate (la-vo), lamotrigine plus lithium (la-li), cautious combination of lamotrigine, lithium, and valproate (la-li-vo), and combination of lithium plus quetiapine (lami-quel). Experts diverge in their opinions on how to treat bipolar depression, particularly when it comes to antidepressants. Some believe that even when combination treatment is required, it should never involve use of an antidepressant (Boston bipolar brew), while others recommend cautious addition of an antidepressant to one or more mood stabilizers (California careful cocktail). For patients who develop symptoms of activation during treatment with an antidepressant for unipolar depression, some experts suggest adding an atypical antipsychotic rather than discontinuing the antidepressant (Tennessee mood shine).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


