Monoclonal Immunoglobulin Deposition Disease
Lynn D. Cornell, MD
Key Facts
Clinical Issues
Nephrotic syndrome
Acute or chronic renal failure
Multiple myeloma diagnosable in ˜ 40% of patients with pure MIDD
Monoclonal protein in serum, urine, or both
˜ 15-20% of patients do not have detectable serum or urine paraprotein at time of diagnosis, even by immunofixation
Microscopic Pathology
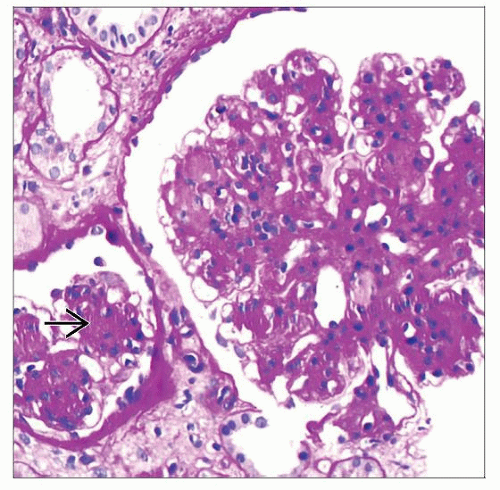
Tubular basement membrane monoclonal immunoglobulin deposition
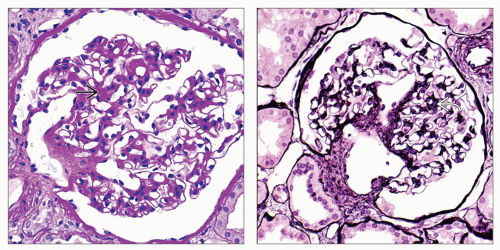
Nodular glomerulopathy
PAS positive nodules, nonargyrophilic
By IF, linear basement membrane staining (glomerular, tubular, vascular) for kappa or lambda light chain (LCDD), plus a heavy chain (LHCDD), or heavy chain only (HCDD)
Kappa light chain in ˜ 80% of LCDD
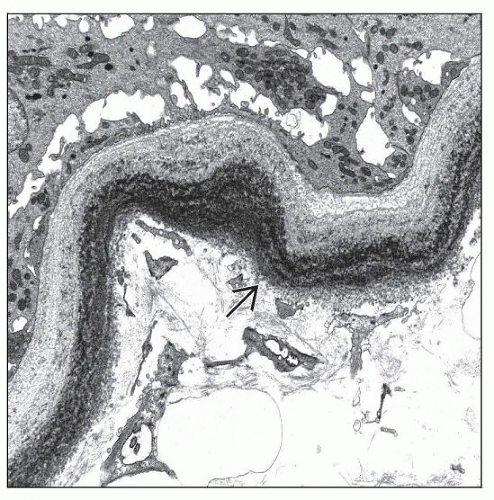
By EM, finely granular, punctate, “powdery” electron-dense deposits distributed along basement membranes; nonfibrillary
Top Differential Diagnoses
Light chain deposition by immunofluorescence only
Light chain tubulointerstitial nephritis
Dense deposit disease (DDD)
Nodular diabetic glomerulosclerosis
Light chain Fanconi syndrome
TERMINOLOGY
Abbreviations
Monoclonal immunoglobulin deposition disease (MIDD)
Synonyms
Systemic light chain disease
Light chain deposition disease (LCDD)
Heavy chain deposition disease (HCDD)
Light and heavy chain deposition disease (LHCDD)
Nonamyloidogenic light chain deposition
Monoclonal immunoglobulin deposition disease, Randall type
Definitions
Deposition of monoclonal immunoglobulin along glomerular and tubular basement membranes (GBMs/TBMs) and within mesangium
Deposits are characterized by linear GBM and TBM staining by immunofluorescence (IF) and finely granular deposits by electron microscopy (EM)
ETIOLOGY/PATHOGENESIS
LCDD Deposits
Differ from normal light chains in variable region, especially complementarity-determining regions and framework regions
Composed of glycosylated light chains, resulting in larger molecule than normal light chain
HCDD Deposits
Deletion of the CH1 constant domain of gamma heavy chain
CLINICAL ISSUES
Presentation
Acute renal failure
Chronic renal failure
Nephrotic syndrome
Proteinuria (58%; non-light chain)
Multiple myeloma diagnosable in ˜ 40% of patients with pure MIDD (without concurrent cast nephropathy or amyloidosis)
Hypocomplementemia
Present in gamma-HCDD with complement-fixing IgG subclass deposited (gamma-1 or gamma-3)
Laboratory Tests
Monoclonal protein in serum, urine, or both
M spike on serum or urine protein electrophoresis in ˜ 50% of patients with pure MIDD
˜ 15-20% of patients do not have detectable serum or urine paraprotein at time of diagnosis, even by immunofixation
Serum or urine free light chain tests can increase sensitivity
Elevated kappa or lambda free light chains in serum or urine
Emerging test for free heavy chains in gamma heavy chain deposition disease
Free light or heavy chain tests useful for monitoring response to therapy
Other Organ Involvement
Cardiac disease (estimated ˜ 19-80%)
Peripheral neuropathy (20%)
Other: Gastrointestinal, liver, pulmonary nodules, muscle, skin
MICROSCOPIC PATHOLOGY
Histologic Features
Nodular glomerulopathy
PAS-positive nodules, nonargyrophilic
Interstitial inflammation
Interstitial edema
Acute tubular injury
TBM thickening may be present
Congo red negative deposits
Concurrent amyloidosis may be present
Rare cases may show thickened glomerular basement membranes with an associated membranoproliferative pattern
These cases must show linear GBM and TBM monoclonal immunoglobulin deposits to be diagnosed as MIDD
Concurrent light chain cast nephropathy present in nearly 1/3 of renal biopsies with MIDD
Concurrent amyloidosis in 13% of MIDD cases
Necrotizing and crescentic glomerulonephritis (rare)
Seen more frequently in alpha-HCDD
ANCILLARY TESTS
Immunohistochemistry
In absence of IF material, cases may show glomerular and TBM deposits for kappa or lambda light chain
Immunofluorescence
Linear basement membrane staining (glomerular, tubular, vascular) for monoclonal immunoglobulin
Usually monotypic kappa light chain in LCDD (73-91% kappa)
Kappa or lambda light chain in LCDD
Heavy chain only in HCDD
Usually IgG
All IgG subclasses (1, 2, 3, and 4) have been reported to cause gamma-HCDD
Rare cases of IgA-HCDD
1 heavy chain and 1 light chain in LHCDD
Smudgy staining of mesangium for monoclonal protein
Staining for IgG subclasses helps determine monoclonal nature of deposits in gamma-HCDD
Electron Microscopy
Finely granular, punctate, “powdery” electron-dense deposits distributed along basement membranes; nonfibrillary
Similar appearing deposits in LCDD, LHCDD, and HCDD
Monoclonal deposits may be seen by immunogold labeling
DIFFERENTIAL DIAGNOSIS
Light Chain Deposition by Immunofluorescence Only
Monotypic light chain staining of GBM and TBM by IF, but no deposits detectable by EM and no changes by light microscopy
Seen especially in cases of light chain cast nephropathy
Uncertain significance
May be artifactual IF staining representative of monoclonal protein in urine
Light Chain Tubulointerstitial Nephritis
Pattern of acute tubulointerstitial nephritis
Absence of a glomerulopathy
Negative IF and routine EM
In some cases, light chain deposits seen in some TBMs by EM with immunogold labeling or by immunoperoxidase staining
Proliferative Glomerulonephritis with Monoclonal IgG Deposits
Dense Deposit Disease (DDD)
Some cases of DDD are associated with serum paraprotein, which may have C3 nephritic factor activity
DDD shows staining by IF for C3 only, not monoclonal immunoglobulins
Very electron-dense deposits along GBMs and in mesangium, distinct from finely granular deposits in GBMs and TBMs in MIDD
Nodular Diabetic Glomerulosclerosis
Similar PAS positive nodular glomerulopathy to MIDD but without IF or EM findings of MIDD
Fibrillary or Immunotactoid Glomerulonephritis
May show monotypic glomerular staining by IF, especially immunotactoid glomerulonephritis
Smudgy IgG kappa or IgG lambda mesangial staining and glomerular basement membrane staining
Fibrillary substructure to deposits by EM
Usual absence of TBM deposits
Type 1 Cryoglobulinemic Glomerulonephritis or Waldenström Macroglobulinemic Glomerulonephritis
Deposition of a monoclonal immunoglobulin in glomeruli
Membranoproliferative pattern of glomerular injury
Absence of finely granular deposits along glomerular and TBMs
Light Chain Fanconi Syndrome (Light Chain Proximal Tubulopathy)
Light chain deposits within tubular epithelial cells rather than within basement membranes
Crystalline deposits within epithelial cytoplasm
Amyloidosis
Congo red positive, amorphous, PAS negative deposits
Fibrillary structure by EM
Amyloid may be present in glomeruli, vessels, and interstitium
IgA Nephropathy
Alpha-HCDD, in part due to its rarity, may be misdiagnosed as IgA nephropathy
Like IgA nephropathy, alpha-HCDD may show necrotizing and crescentic glomerulonephritis
By IF, alpha-HCDD shows linear TBM staining for IgA along with glomerular staining
By EM, alpha-HCDD shows granular GBM and TBM deposits typical of MIDD
Usual absence of TBM deposits in IgA nephropathy
Recurrent or De Novo MIDD in Allograft
May be seen on protocol biopsies when clinically inapparent
DIAGNOSTIC CHECKLIST
Clinically Relevant Pathologic Features
31-45% of patients with pure MIDD have overt multiple myeloma at time of MIDD diagnosis
91% of patients with concurrent MIDD and cast nephropathy have multiple myeloma
M spike on serum or urine protein electrophoresis in ˜ 50% of patients with pure MIDD
Hypocomplementemia
Present in gamma-HCDD with complement-fixing IgG subclass deposited (gamma-1 or gamma-3)
SELECTED REFERENCES
1. Herrera GA et al: Ultrastructural immunolabeling in the diagnosis of monoclonal light-and heavy-chain-related renal diseases. Ultrastruct Pathol. 34(3):161-73, 2010
2. Sethi S et al: Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am J Kidney Dis. 56(5):977-82, 2010
3. Alexander MP et al: Alpha heavy chain deposition disease: a comparison of its clinicopathological characteristics with gamma and mu heavy chain deposition disease. Mod Pathol. 20(Suppl. 2):270A, 2007
4. Salant DJ et al: A case of atypical light chain deposition disease—diagnosis and treatment. Clin J Am Soc Nephrol. 2(4):858-67, 2007
5. Gu X et al: Light-chain-mediated acute tubular interstitial nephritis: a poorly recognized pattern of renal disease in patients with plasma cell dyscrasia. Arch Pathol Lab Med. 130(2):165-9, 2006
6. Rosenstock JL et al: Fibrillary and immunotactoid glomerulonephritis: Distinct entities with different clinical and pathologic features. Kidney Int. 63(4):1450-61, 2003
7. Lin J et al: Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J Am Soc Nephrol. 12(7):1482-92, 2001
8. Buxbaum J et al: Nonamyloidotic monoclonal immunoglobulin deposition disease. Light-chain, heavy-chain, and light- and heavy-chain deposition diseases. Hematol Oncol Clin North Am. 13(6):1235-48, 1999
9. Kambham N et al: Heavy chain deposition disease: the disease spectrum. Am J Kidney Dis. 33(5):954-62, 1999
10. Cheng IK et al: Crescentic nodular glomerulosclerosis secondary to truncated immunoglobulin alpha heavy chain deposition. Am J Kidney Dis. 28(2):283-8, 1996
11. Buxbaum J: Mechanisms of disease: monoclonal immunoglobulin deposition. Amyloidosis, light chain deposition disease, and light and heavy chain deposition disease. Hematol Oncol Clin North Am. 6(2):323-46, 1992
12. Randall RE et al: Manifestations of systemic light chain deposition. Am J Med. 60(2):293-9, 1976
Image Gallery
Light Chain Deposition Disease
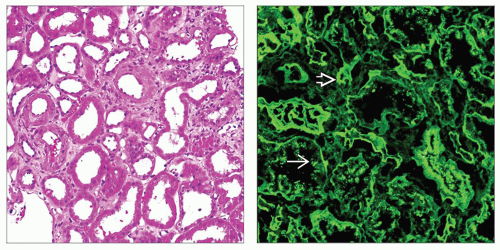 (Left) Tubules are dilatated and show flattening of the epithelium, both features of acute injury. This patient had proteinuria and acute renal failure. Tubular symptoms, such as polyuria, can dominate in light chain MIDD. (Right) IF staining reveals bright linear tubular basement membrane staining for kappa light chain
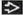 , the most common light chain in MIDD, with negative staining for lambda light chain (not shown). Tubular protein reabsorption droplets are also present and stain for kappa , the most common light chain in MIDD, with negative staining for lambda light chain (not shown). Tubular protein reabsorption droplets are also present and stain for kappa  . .Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|