S. N.
Property
Conventional antiserum
Monoclonal antibody
1.
Determinant
Several
Single
2.
Specificity
Variable with animal and bleed
Standard
Partial cross-reactions with common determinants
Unexpected cross-reactions may occur
Seldom too specific
May be too specific for requirements
3.
Affinity
Variable with bleed
May be selected during cloning
4.
Yield of useful antibody
Up to 1 mg/ml
Up to 100 μg/ml in tissue culture, Up to 20 mg/ml in ascitic fluid
5.
Contaminating Immunoglobulin
Up to 100 %
None in culture, 10 % in ascetic fluid
6.
Purity of antigen
Either pure antigen or serum absorption
Some degree of antigen purification desirable but not essential.
7.
Approx. Minimum cost
Usually below £100
Capital cost £10,000, Running cost £10,000 p.a.
11.2 Production of Monoclonal Antibodies
Antibodies are secreted by plasma cells which are programmed in lymph glands for producing antibodies against a particular epitope. The plasma cells can be cultured and made to secrete antibodies but they have a very short life span and die soon. The technique developed by Kohler and Milstein (1975) immortalizes the plasma cells which can thus produce monoclonal antibodies indefinitely. For this purpose they fused the plasma cells with cancerous cells called myeloma cells. Myeloma cells can be obtained from animals suffering from myeloma tumors or tumors can be induced in experimental animals by injecting mineral oil. They used an inbred strain of mice known as Balb/c mice for this purpose. A schematic illustration for monoclonal antibody production is given in Fig. 11.1. All the steps are also detailed subsequently.
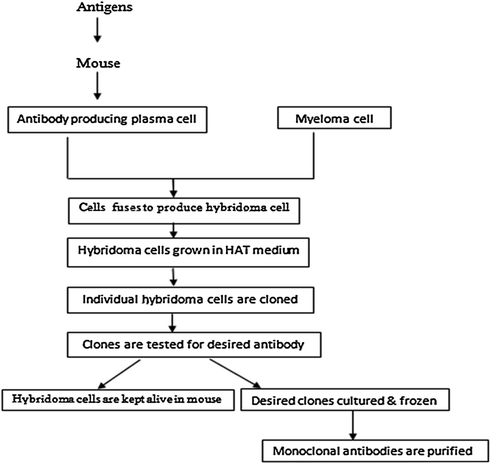

Fig. 11.1
Schematic representation of monoclonal antibody production
11.3 Monoclonal Antibody Assay Requirements
Assay is the most critical factor in production of good hybridomas and is very sensitive. Understanding of the theoretical background is essential for the production of a large number of hybridomas secreting antibody of the required characteristics.
a.
Rate of association
The initial rate of association of an antibody with an antigen is described by the equation:
![$$ {\text{Rate}}\;{\text{of}}\;{\text{formation}}\;{\text{of}}\;{\text{product}}\;
= \;{\text{K}}_{ 1} [{\text{antibody}}]{\text{[antigen]}} $$](/wp-content/uploads/2016/10/A315564_1_En_11_Chapter_Equa.gif)
![$$ {\text{Rate}}\;{\text{of}}\;{\text{formation}}\;{\text{of}}\;{\text{product}}\;
= \;{\text{K}}_{ 1} [{\text{antibody}}]{\text{[antigen]}} $$](/wp-content/uploads/2016/10/A315564_1_En_11_Chapter_Equa.gif)
The number of epitopes on the antigen is much reduced and may be one in case of small protein. Thus the effective concentration of the antigen is quite low. The antibody will still be bivalent or decavalent for IgM but its concentration may be very low. The association rate is usually the parameter which determines the final equilibrium constant and the concentration of antigen in most assays remains constant.
b.
Rate of dissociation
The rate of dissociation of an antibody-antigen complex is represented by the following equation:
![$$\text{Initial}\;{\text{rate}}\;{\text{of}}\;{\text{dissociation}}\;
= \;{\text{K}}_{ 2} ([{\text{antibody} - {\text{antigen}}}])$$](/wp-content/uploads/2016/10/A315564_1_En_11_Chapter_Equb.gif)
![$$\text{Initial}\;{\text{rate}}\;{\text{of}}\;{\text{dissociation}}\;
= \;{\text{K}}_{ 2} ([{\text{antibody} - {\text{antigen}}}])$$](/wp-content/uploads/2016/10/A315564_1_En_11_Chapter_Equb.gif)
The dissociation rate is nearly always very much lower than the association rate. With most antibody-antigen reactions, it is the dissociation rate rather than the association rate that determines the affinity as the dissociation rate can vary over 8–9 orders of magnitude. The dissociation rate is not only the most variable among different antibodies under a defined set of conditions but also the most variable in a single antibody with respect to environmental conditions such as pH, temperature, etc.
c.
Equilibrium concentration of reactants
The equilibrium constant for an antibody-antigen reaction is the ratio of the forward to the backward rates i.e.,
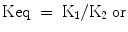
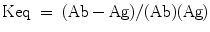
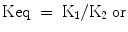
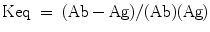
The concentration of the reactants and the assay conditions may influence the possibility of detection of a suitable hybridoma. With polyclonal sera it can be assumed that an optimal equilibrium condition will be achieved by incubation for one or two hours at room temperature, biological pH, and ionic strength, etc. but for monoclonal antibodies several of the parameters will have to be varied and screening procedures will have to be extended if antibodies to more interesting or relevant epitopes are to be detected.
d.
Effect of multivalence
All antibodies are at least divalent and IgA and IgM antibodies may have several idiotypes on the same molecule. Multivalent antibodies are helpful to screening procedures since the effective dissociation rate can be reduced if enough antigens are present. Antigens having multiple epitopes, mostly found in bacterial systems with symmetrical cell wall structures, can affect the reaction in many ways.
e.
Specificity and affinity
Monoclonal antibodies against polymorphic antigens could exhibit extreme specificity in one assay and considerable epitope overlap in a second higher affinity assay. However, it is assumed that at least in the initial stages, an assay detecting the maximum numbers of positive clones is required. Suitable conditions for obtaining specific responses can then be achieved later.
f.
Number of assays
In a typical fusion using 4 × 96 well plates some 400 samples must be assayed in a short time. Subcloning usually involves a similar number although a valuable clone may be subcloned with more. Further, a comprehensive screen of hybridomas should ideally involve the use of several plates under different sets of conditions. If the final application of the antibody is not considered then positive samples detected by the first screening should be assayed by a second directed to the final application, since the number of positive clones is much lower than the total number of clones.
g.
Time of assay
The time of assay, in terms of clonal growth, should be soon after clones are microscopically visible and again a few days after when the clones are visible to the eye. Screening should continue for several weeks. The actual assay however, is influenced by the antibody concentration.
h.
pH of assay
It is expected that the most antibodies have their optimal reaction with antigen at physiological pH. It is quite possible to fail to detect a good hybridoma by screening at a single pH. The pH of tissue culture fluid in which cells are growing can vary almost a whole pH unit. If a defined pH is required for the final application then the assay should be buffered accordingly during the incubation of antibody with antigen, particularly for in vivo uses. Where pH adjustments are necessary the nature of the buffer should be considered as the buffer components themselves may affect the assay.
i.
Temperature of assay
Dissociation constant is the variable which is most sensitive to temperature. The best assay conditions for hybridoma favor incubation at 4 °C rather than at room temperature or higher but ideally both should be tried unless the final use precludes certain temperature.
j.
Ionic strength of assay
There is no detailed information on the ionic strength variations in hybridoma assays. Nonspecific binding is more likely to occur at low ionic strengths. If different pH buffers are used in the assay then all buffers used should have the same ionic strength.
11.4 Cell Culture Requirements for Hybridomas
1.
Media
Two main types of media used for hybridoma production are Dulbecco’s Modification of Eagles Medium (DMEM) and Rosewell Park Memorial Institute (RPMI) medium. Media are prepared in double distilled water, sterilized by filtration through 0.2 μm membranes, and usually stored in 500–1,000 ml aliquots for up to 6 weeks. Both the above media are bicarbonate buffered with phenol red indicator. The correct color for medium is bright orange indicating a pH of 7.2.
DMEM or RPMI 1640 supplemented with high glucose (4.58 g/l) are popularly used for culturing myeloma cells. Glutamine (2 mM final concentration), antibiotics such as penicillin (100 U/ml) or streptomycin (10 μg/ml) and 10 % fetal calf serum is also added. Myeloma cells should be grown in the presence of 8-azaguanine prior to fusion to ensure HGPRT negative character. For fusion, cells should be in logarithmic phase (3–8 × 106/ml).
2.
Sera
Fetal calf serum (FCS) is used in nearly all hybridoma work because of the low level of contaminating immunoglobulin. It is kept frozen at −20 °C. Horse and rabbit serum is also sometimes used.
3.
Antibiotics for prevention of contamination
The main antibiotics used in hybridoma production are penicillin and streptomycin. Penicillin inhibits the growth of most gram-positive bacteria, whereas streptomycin inhibits the growth of most gram-negative bacteria. The antibiotic preparations are usually made up in 100 × stock solutions containing 107 units of sodium benzyl penicillin and 10 g of streptomycin sulfate per liter. This is filter sterilized through 0.2 μm membranes and stored in 20 ml aliquots at −20 °C. Some labs use fungizone (Amphotericin B) at 2.5 μg/ml medium.
Mycoplasma contamination may be prevented by kanamycin (100 μg/ml), tylocine (50 μg/ml) or lincomycin, and vanomycin to some extent. Exposure of cells at elevated temperatures has also been reported to curb mycoplasma contamination.
Viral contamination with Epstein Barr (EB) virus in a latent form is usually present if human lymphocytes are used. The virus transforms culture especially in the absence of cytotoxic T lymphocytes. Lymphocyte donors are usually tested for antibody to the viral capsid antigen by use of cell line P3HR1which secretes non-transforming EB.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


