Molecular Testing of Thyroid FNA Samples
Marina N. Nikiforova
Yuri E. Nikiforov
Molecular testing of thyroid fine needle aspiration (FNA) samples is gaining its use as an ancillary diagnostic tool for cancer diagnosis in thyroid nodules. Palpable thyroid nodules are common in adults and estimated to affect >100 million people in the United States.1,2 Most of thyroid nodules are benign and can be managed conservatively, whereas approximately 5% to 15% of medically evaluated thyroid nodules are malignant.3,4,5,6,7 A clinical challenge facing the physician is to accurately diagnose cancer in these nodules to ensure that each patient receives timely and appropriate treatment, while minimizing the risk of unnecessary thyroid surgery for benign disease.
Currently, the most common and reliable diagnostic tool for the evaluation of thyroid nodules is FNA cytology. FNA provides a definitive diagnosis of benign or malignant thyroid disease in most cases. However, in about 25% of nodules, FNA cytology cannot reliably exclude cancer and such cases are placed in one of the indeterminate categories.1,3,8,9 This is because cytologic features of thyroid lesions with a follicular growth pattern are frequently not sufficiently different to distinguish between benign and malignant lesions.
The current Bethesda System for Reporting Thyroid Cytopathology recognizes three types of indeterminate cytological diagnosis: (1) atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), (2) follicular neoplasm/suspicious for follicular neoplasm (FN/SFN), and (3) suspicious for malignant cells (SMC), with a predicted probability of cancer of 5% to 15%, 15% to 30%, and 60% to 75%, respectively.10,11 Because FNA is unable to provide a definitive diagnosis for these nodules, most patients with indeterminate cytology undergo diagnostic surgery to establish a histopathologic diagnosis. However, only 10% to 40% of such surgically resected thyroid nodules will prove to be malignant.4,11,12 The unneeded operations can be avoided if an FNA procedure allowed to reliably establish the diagnosis of a benign nodule. For patients diagnosed with cancer after lobectomy, the standard of care is to offer a second operation to complete the thyroidectomy, with additional costs and increased morbidity. The improved diagnosis of cancer established preoperatively can lead to more optimal surgical treatment with a single “up-front” total thyroidectomy. Furthermore, BRAF V600E mutation has been associated with a substantially worse outcome, and nodal metastasis and the availability of this information preoperatively may be helpful to define the extent of surgery. Diagnostic use of mutational markers for thyroid FNA samples has been explored for a panel of mutations and for single genes.
Table 21.1 Probability of cancer in thyroid nodules positive for specific mutations based on clinical studies that utilized a panel of mutations | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
TESTING FOR PANEL OF MUTATIONS
Testing for a panel of mutations is feasible in thyroid FNA samples and provides helpful diagnostic and prognostic information.13,14,15,16 The panel includes most common mutations that collectively occur in approximately 70% of thyroid cancer, i.e. BRAF V600E, BRAF K601E, NRAS codon 61, HRAS codon 61, and KRAS codons 12/13 point mutations and RET/PTC1, RET/PTC3, and PAX8/PPARγ rearrangements, with the possible addition of the TRK rearrangement. The results of large and well-designed prospective studies performed in clinical molecular diagnostic laboratories demonstrate that detection of any mutation in an aspirated thyroid nodule was a strong predictor of malignancy irrespective of the cytological diagnosis.13,14,15,16 Specifically, the presence of BRAF, RET/PTC, or PAX8/PPARγ was specific for malignant outcome in 100% of cases, whereas RAS mutations had a 74% to 100% positive predictive value for cancer (Table 21.1).
Testing for the panel of mutations is particularly helpful in nodules with indeterminate cytology, where it improves an
assessment of cancer risk. In a prospective study of >1,000 thyroid FNA samples, identification of a mutation in specific categories of indeterminate cytology, that is, AUS/FLUS, FN/SFN, and SMC, conferred the risk of histologic malignancy of 88%, 87%, and 95%, respectively (Fig. 21.1).13 The most common mutations found in these nodules were RAS, followed by BRAF, and PAX8/PPARγ, and the most frequent tumors carrying these mutations were follicular variant papillary carcinomas and follicular carcinomas, which are difficult to diagnose by FNA cytology. The risk of cancer in mutation-negative nodules was 6% in AUS/FLUS samples, 14% in FN/SFN, and 28% in SMC (Fig. 21.1).13 In the AUS/FLUS group, among nodules negative for mutations, only 2.3% of cancers were invasive and only 0.5% had extrathyroidal extension.13 Based on these data, combination of FNA cytology and molecular testing can result in a more refined clinical management of patients with thyroid nodules (Fig. 21.1).13 Indeed, positive results of molecular testing, with the possible exception of RAS-positive nodules, should offer a strong indication for total thyroidectomy in all categories of indeterminate cytology. This will allow bypassing the repeat of FNA and eliminate the need for a two-step surgery, that is, diagnostic lobectomy followed by completion thyroidectomy, for most patients with malignant nodules.
assessment of cancer risk. In a prospective study of >1,000 thyroid FNA samples, identification of a mutation in specific categories of indeterminate cytology, that is, AUS/FLUS, FN/SFN, and SMC, conferred the risk of histologic malignancy of 88%, 87%, and 95%, respectively (Fig. 21.1).13 The most common mutations found in these nodules were RAS, followed by BRAF, and PAX8/PPARγ, and the most frequent tumors carrying these mutations were follicular variant papillary carcinomas and follicular carcinomas, which are difficult to diagnose by FNA cytology. The risk of cancer in mutation-negative nodules was 6% in AUS/FLUS samples, 14% in FN/SFN, and 28% in SMC (Fig. 21.1).13 In the AUS/FLUS group, among nodules negative for mutations, only 2.3% of cancers were invasive and only 0.5% had extrathyroidal extension.13 Based on these data, combination of FNA cytology and molecular testing can result in a more refined clinical management of patients with thyroid nodules (Fig. 21.1).13 Indeed, positive results of molecular testing, with the possible exception of RAS-positive nodules, should offer a strong indication for total thyroidectomy in all categories of indeterminate cytology. This will allow bypassing the repeat of FNA and eliminate the need for a two-step surgery, that is, diagnostic lobectomy followed by completion thyroidectomy, for most patients with malignant nodules.
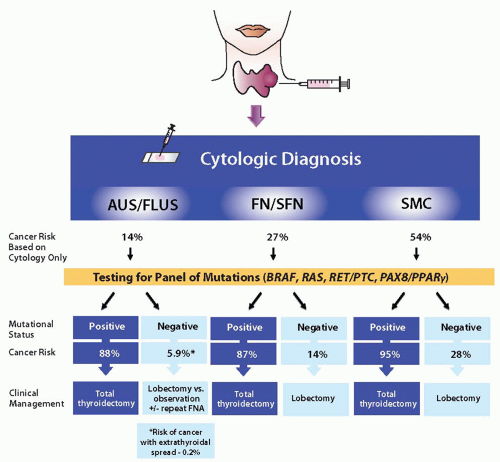 FIGURE 21.1 Cancer risks and algorithm for management of patients with cytologically indeterminate thyroid FNAs based on the results of mutational analysis combined with specific cytologic diagnosis. |
The mutation negative result does not completely eliminate the risk of cancer. Therefore, diagnostic lobectomy appears to be justified as initial surgical intervention for mutation-negative nodules with FN/SFN and SMC cytology. The appropriate approach for nodules with AUS/FLUS cytology that are negative for mutations is less clear. A 6% cancer risk in these nodules, with a risk of cancer extending outside the thyroid gland of <1%, raise a possibility of a more conservative management with clinical and ultrasound follow-up and repeat of FNA in appropriately selected patients.
The 2009 American Thyroid Association’s management guidelines recommend to consider the mutational panel for nodules with indeterminate FNA cytology to help guide clinical management.17
TESTING FOR BRAF MUTATION
BRAF V600E is a highly specific marker for cancer diagnosis in thyroid FNA samples. A meta-analysis of the results reported in
22 studies15,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 of thyroid FNA samples tested for BRAF V600E revealed that out of 1,117 nodules found positive for this mutation, 1,109 (99.3%) were diagnosed as papillary carcinomas on final histopathology.38 Eight cases were false-positive as no cancer was found after surgery, of which five cases were reported in one study that used ultrasensitive detection of this mutation.37 Even if these cases are accepted as true false-positive, BRAF mutation appears to be a highly accurate marker of cancer in thyroid nodules sampled by FNA, conferring the risk of malignancy above 99%. Importantly, 15% to 40% of samples tested positive for BRAF had indeterminate FNA cytology, indicating that BRAF can be of significant diagnostic value in these nodules.15,25,29,32,35,37,39 Despite high specificity for cancer, testing for BRAF mutation alone misses many thyroid cancers that are negative for this mutation. The performance of molecular testing can be significantly improved by including in the analysis other frequently occurring mutations.
22 studies15,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 of thyroid FNA samples tested for BRAF V600E revealed that out of 1,117 nodules found positive for this mutation, 1,109 (99.3%) were diagnosed as papillary carcinomas on final histopathology.38 Eight cases were false-positive as no cancer was found after surgery, of which five cases were reported in one study that used ultrasensitive detection of this mutation.37 Even if these cases are accepted as true false-positive, BRAF mutation appears to be a highly accurate marker of cancer in thyroid nodules sampled by FNA, conferring the risk of malignancy above 99%. Importantly, 15% to 40% of samples tested positive for BRAF had indeterminate FNA cytology, indicating that BRAF can be of significant diagnostic value in these nodules.15,25,29,32,35,37,39 Despite high specificity for cancer, testing for BRAF mutation alone misses many thyroid cancers that are negative for this mutation. The performance of molecular testing can be significantly improved by including in the analysis other frequently occurring mutations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


