Fig. 1
Schematic representation of desirable sites of action of antipruritic agents. Since vigorous scratching worsens skin conditions, suppression of both itching and scratching is important in the treatment of chronic pruritic diseases. Scratching is not only induced by an itching sensation but also mediated by a spinal reflex. Therefore, in order to suppress itch-associated scratching, antipruritic agents should inhibit the generation of itch signals in the skin or the synaptic transmission of itch signals in the dorsal horn. Various itch mediators are involved in the generation of itch in the skin, whereas only a few groups of dorsal horn neurons selectively receive itch signals from the skin. Therefore, pharmacological modulation of itch transmission in the dorsal horn is an effective way to suppress both itching and scratching in pruritic diseases
2 Central Regulation of Itch Sensation
2.1 Counterirritants
Noxious counterstimuli applied to skin areas adjacent to the itch site suppress itch perception (Ward et al. 1996). The antipruritic state produced by brief noxious stimuli persists for longer than 30 min (Ward et al. 1996). Subpopulations of wide-dynamic-range (responsible for all somatosensory modalities and a wide range of intensity of peripheral stimulation) and nociceptive-specific neurons in the superficial dorsal horn receive itch signals from the skin (Andrew and Craig 2001; Jinks and Carstens 2002; Omori et al. 2009). Primate spinothalamic tract neurons and murine superficial dorsal horn neurons respond to an intradermal injection of histamine, a pruritogen, and these responses are inhibited by scratching of the receptive field, a counterstimulus (Davidson et al. 2009; Akiyama et al. 2012). In contrast, scratching does not inhibit the responses of these neurons to painful stimuli (Davidson et al. 2009; Akiyama et al. 2012). Scratching within an area 5–17 mm distant from the injection site inhibits the pruriceptive response, but scratching of the injection site does not (Akiyama et al. 2012). Thus, scratching exerts site- and state-dependent inhibition of pruritogen-responsive spinal neurons.
Superficial dorsal horn neurons in mice with pruritic dry skin exhibit high levels of spontaneous firing that is inhibited by scratching, pinching, and noxious heat stimulation (Akiyama et al. 2011). Scratch-induced inhibition is suppressed by spinal administration of strychnine (a glycine receptor antagonist), bicuculline (a GABAA receptor antagonist), and saclofen (a GABAB receptor antagonist) (Akiyama et al. 2011). These findings suggest that glycinergic and GABAergic interneurons are involved in the inhibition of the pruriceptive responses of the dorsal horn neurons induced by noxious counterstimuli. In addition, scratch-induced inhibition is attenuated by cold block and transection of the upper spinal cord, suggesting that descending pathways from the brain are also involved in the inhibition of pruriceptive responses (Akiyama et al. 2011).
Lowering skin temperature from 33 °C to 30 °C by innocuous cooling reduces the intensity of histamine-induced itch; moreover, topical application of menthol reduces itch intensity, although skin temperature does not decrease (Bromm et al. 1995). These temporal reductions in itch may be mediated by activating receptor channels of the transient receptor potential family, particularly transient receptor potential M8 channel (Peier et al. 2002; Story et al. 2003). Inhibition of itch-related behaviors by menthol has been shown to be mediated by a subpopulation of inhibitory interneurons (B5-I neurons) in the dorsal horn (Kardon et al. 2014).
2.2 Distraction
Innocuous stimulation, such as vibration and warming, of skin areas adjacent to the itch site reduces histamine-induced itch (Ward et al. 1996). Vibration of the opposite side of the body also reduces itch (Melzack and Schecter 1965). In addition, these innocuous stimuli mildly reduce mustard oil-induced pain (Ward et al. 1996). These inhibitions do not persist for longer than 20 s when the counterstimuli are removed and may be because of distraction, a state in which attention is diverted from the central part of an experience (Ward et al. 1996). Distraction with noncontact stimulation also inhibits itching in humans (Leibovici et al. 2009) and scratching behaviors in mice (Tohda et al. 1997; Yamaguchi et al. 2001).
3 Noradrenergic Inhibition of Itch Transmission
3.1 Descending Noradrenergic System
Depletion of spinal noradrenaline by intrathecal injection of 6-hydroxydopamine, the catecholaminergic neurotoxin, increases itch-related behavior, including biting of the affected area of the hind paw (Hagiwara et al. 1999; Gotoh et al. 2011b; LaMotte et al. 2011), induced by acute cutaneous allergy and intradermal pruritogen injection in mice (Gotoh et al. 2011b, c). The itch-related response is inversely correlated with noradrenaline content in the spinal cord in mice that are treated intrathecally with 6-hydroxydopamine (Gotoh et al. 2011b). Intrathecal injection of phentolamine, the nonselective α-adrenoceptor antagonist, also increases itch-related behavior induced by acute allergy and pruritogen injection (Gotoh et al. 2011b, c). These findings suggest that noradrenaline is an itch-inhibiting transmitter in the spinal cord and that itch signaling is under tonic inhibitory control of the descending noradrenergic system, which has been long known as a pain-inhibiting system. In contrast, depletion of spinal serotonin, another monoaminergic transmitter of the descending pain-inhibiting system, by intrathecal injection of 5,7-dihydroxytryptamine, the serotonergic neurotoxin, does not increase itch-related behavior induced by acute cutaneous allergy (Gotoh et al. 2011c) and pruritogen injection (unpublished observation).
In rats, wide-dynamic-range neurons in the superficial dorsal horn respond to intradermal histamine injection within the receptive field; however, these neurons respond to algogenic stimuli as well (Jinks and Carstens 2002). Electrical stimulation of the midbrain periaqueductal gray suppresses the responses of these neurons to histamine (Carstens 1997). Electrical stimulation of the midbrain periaqueductal gray also increases the release of noradrenaline in the spinal cord (Cui et al. 1999). Noradrenaline-containing neurons are not present in the midbrain periaqueductal gray, which densely innervates A6 (locus coeruleus) and A7 catecholamine cell groups (Bajic and Proudfit 1999). Electrical stimulation in the locus coeruleus and at sites near the A7 cell group inhibits nociception mediated by descending noradrenergic systems at the spinal cord level (Jones and Gebhart 1986; Yeomans et al. 1992). It is possible that these noradrenergic pain-inhibiting pathways are also involved in inhibiting itch signaling in the spinal cord (Fig. 2). Noradrenergic terminals have hardly any synaptic contacts with central terminals of primary sensory neurons and with dorsal horn neurons, and volume transmission (transmitter diffusion to the target cells in the extracellular space) has been suggested to have an important role in the noradrenergic modulation of neuronal activity (Hagihira et al. 1990; Rajaofetra et al. 1992). Therefore, itch and pain transmission may be modulated by common noradrenergic pathways. Neurons in the locus coeruleus exhibit spontaneous action potential discharge in vivo (Sugiyama et al. 2012), suggesting that noradrenaline is released spontaneously from the axon terminals of noradrenergic neurons in the locus coeruleus. This raises the possibility that noradrenergic neurons in the locus coeruleus play a role in the tonic inhibitory control of itch signaling in the spinal cord.
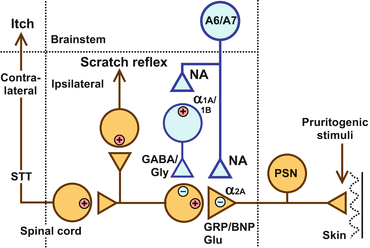

Fig. 2
Schematic illustration of the itch-signaling pathway and its inhibition by the descending noradrenergic system. Itch signals that originate in primary sensory neurons (PSN) are transmitted to interneurons in the dorsal horn by glutamate (Glu) and gastrin-releasing peptide (GRP)/B-type natriuretic peptide (BNP). Itch signals are further conveyed to the contralateral brain by spinothalamic tract (STT) neurons to form an itch sensation. Itch signals conveyed in the ipsilateral white column evoke the scratch reflex. The descending noradrenergic system that inhibits itch transmission in the dorsal horn probably originates from A6 (locus coeruleus) and A7 catecholamine cell groups. The antipruritic activity of noradrenaline (NA) may be mediated by inhibitory α2A-adrenoceptors presynaptically located on the central terminals of primary afferents and excitatory α1A– and α1B-adrenoceptors postsynaptically located on the inhibitory interneurons, which use γ-aminobutyric acid (GABA) or glycine (Gly) as a neurotransmitter
3.2 α1-Adrenoceptors
Although pruritogen-induced itch-related behavior increases upon intrathecal injection of phentolamine, it is not affected by an intrathecal injection of the α1-adrenoceptor antagonist, prazosin, or the α2-adrenoceptor antagonist, yohimbine (Gotoh et al. 2011b). Therefore, α1– and α2-adrenoceptors may play significant roles in noradrenergic inhibition in the spinal cord. Itch-related behavior induced by intradermal pruritogen injection is inhibited by intrathecal injections of the α1-adrenoceptor agonist phenylephrine and the α2-adrenoceptor agonist clonidine. In fact, phenylephrine and clonidine cause almost complete inhibition at doses that do not affect locomotor activity (Gotoh et al. 2011a, b). These findings support the idea that both α1– and α2-adrenoceptors are involved in inhibitory regulation of itch signaling in the spinal cord (Fig. 2). The antipruritic action of phenylephrine is antagonized by intrathecal treatment with prazosin, the α1-adrenoceptor antagonist (Gotoh et al. 2011b), which has similar binding affinities for α1A-, α1B-, and α1D-adrenoceptors (Bylund et al. 1994). However, it is not antagonized by intrathecal treatment with the α1-adrenoceptor subtype-selective antagonists 5-methylurapidil, cyclazosin, and BMY 7378 (Gotoh et al. 2011b), which have relatively high binding affinities for α1A-, α1B-, and α1D-adrenoceptors, respectively. α1A– and α1B-adrenoceptor mRNAs are expressed in the spinal dorsal horn and dorsal root ganglion; however, α1D-adrenoceptor mRNA is not expressed in these regions in rodents (Nicholson et al. 2005; Gotoh et al. 2011b). Taken together, these findings suggest that α1A– and α1B-adrenoceptors are involved in the noradrenergic regulation of itch signaling (Fig. 2).
Noradrenaline and phenylephrine directly excite GABAergic and glycinergic inhibitory interneurons in the spinal lamina II (substantia gelatinosa) (Baba et al. 2000a). Prazosin and the selective α1A-adrenoceptor antagonist WB 4101 antagonize the excitatory action of phenylephrine (Baba et al. 2000b; Gassner et al. 2009). Such a mechanism is likely to be involved in α1-adrenoceptor-mediated inhibition of itch signaling in the superficial dorsal horn (Fig. 2).
3.3 α2-Adrenoceptors
There are three types of α2-adrenoceptors: α2A-, α2B-, and α2C-receptors. The antipruritic action of intrathecally administered clonidine is almost completely antagonized by intrathecal injection of yohimbine, the α2-adrenoceptor antagonist (Gotoh et al. 2011a), which has similar binding affinities for α2A-, α2B-, and α2C-adrenoceptors (Uhlen et al. 1994). However, it is not inhibited by prazosin (Gotoh et al. 2011a), the α1-adrenoceptor antagonist with substantial binding affinities for α2B– and α2C-adrenoceptors (Bylund et al. 1994). Therefore, α2A-adrenoceptors may play an important role in the antipruritic action of clonidine (Fig. 2). The α2A-adrenoceptors also have a major role in the pain-suppressive effect induced by α2-adrenergic agents (Pertovaara 2006). α2A-Adrenoceptors are located presynaptically on the central terminals of capsaicin-sensitive sensory neurons (Stone et al. 1998). Neurons expressing the BB2 bombesin receptor (a receptor for gastrin-releasing peptide) or the natriuretic peptide receptor A, on which B-type natriuretic peptide mainly acts in the dorsal horn, may play key roles in itch signaling in the superficial dorsal horn (Sun et al. 2009; Mishra and Hoon 2013). Primary afferent C-fiber-evoked responses of the superficial dorsal horn neurons that respond to gastrin-releasing peptide are blocked by antagonists of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and kainate glutamate receptors, suggesting that glutamate plays a major role in itch signaling (Koga et al. 2011). Capsaicin-induced glutamate release from the dorsal horn in vitro is suppressed by clonidine, and this effect is antagonized by yohimbine (Ueda et al. 1995). Inhibition of the release of transmitter(s) from the central terminals of primary afferents may be a mechanism underlying α2-adrenoceptor-mediated noradrenergic inhibition of itch signaling in the dorsal horn (Fig. 2).
4 Systemic Pharmacological Agents
4.1 α-Adrenoceptor Agonists
Intraperitoneal administration of clonidine suppresses pruritogen-induced itch-related behavior: doses of 1–10 μg/kg produce dose-dependent inhibition with nearly complete inhibition at 10 μg/kg (Gotoh et al. 2011a). Intraperitoneal clonidine injection at a dose of 100 μg/kg causes almost complete inhibition of acetic acid-induced pain-related behaviors in mice, whereas 10 μg/kg causes partial inhibition (Bezerra et al. 2008). Thus, antipruritic doses of clonidine are lower than antinociceptive doses. Itch-related behavior is inhibited by intrathecal but not intraplantar nor intracisternal clonidine injections, and the antipruritic effect of intraperitoneal clonidine is almost completely reversed by intrathecal yohimbine (Gotoh et al. 2011a). It is suggested that the antipruritic effect of clonidine is predominantly mediated by α2-adrenoceptor stimulation in the dorsal horn.
4.2 Noradrenaline Reuptake Inhibitors
Tonic inhibition of spinal itch processing by the descending noradrenergic system raises the possibility that antidepressants that block noradrenaline reuptake may exert inhibitory effects against cutaneous pruritus. As expected, pruritogen-induced itch-related behavior in mice is suppressed by intrathecal injection of milnacipran, a serotonin–noradrenaline reuptake inhibitor. In contrast, it is not inhibited by intrathecal injection of fluvoxamine, a selective serotonin reuptake inhibitor (Andoh et al. 2013). Intraperitoneal administration of milnacipran also inhibits itch-related behavior, which is almost completely reversed by intrathecal injection of phentolamine (Andoh et al. 2013). Thus, agents that inhibit noradrenaline reuptake represent potential spinal-acting antipruritic drugs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


