and Alena Skalova2
(1)
Departamento de Ciências Biomédicas e Medicina, Universidade do Algarve, Faro, Portugal
(2)
Department of Pathology, Medical Faculty Charles University, Plzen, Czech Republic
16.1 Translocation-Associated Salivary Gland Tumours
Chromosome rearrangements, in particular translocations, may result in fusion oncogenes encoding oncoproteins with transforming activity [99, 101, 107]. Translocations are believed to account for about 20 % of all cancers [107]. Until recently, majority of translocations and their resultant fusion oncogenes were found in sarcomas and in leukaemias and only few in solid tumours [59, 100]. Five salivary gland tumours are currently known to harbour translocations and fusion oncogenes, namely, pleomorphic adenoma (PA), mucoepidermoid carcinoma (MEC), adenoid cystic carcinoma (ADCC), mammary analogue secretory carcinoma (MASC) and hyalinising clear cell carcinoma (HCCC) [93, 107].
16.1.1 Pleomorphic Adenoma and Carcinoma Ex Pleomorphic Adenoma
Pleomorphic adenoma is the most common histological subtype of salivary gland tumour. It is a benign tumour with a highly variable morphology that sometimes may cause diagnostic problems. Extensive cytogenetic studies of pleomorphic adenomas have shown that they are characterised by recurrent translocations or chromosomal rearrangements with breakpoints preferentially affecting 8q12 (50 % of the cases) and 12q14–15 (10–15 % of the cases) [99, 100]. The translocations/rearrangements result in gene fusions involving the transcription factor genes PLAG1 and HMGA2 [29, 41]. PLAG1 encodes a developmentally regulated DNA-binding zinc finger protein that is part of a family of cell cycle progression-related proteins. HMGA2 belongs to the high mobility group (HMG) protein gene family which encodes proteins that are heterogeneous, nonhistone components of chromatin [99, 100]. The PLAG1 and HMGA2 fusions in pleomorphic adenoma have not been encountered in any other histopathologic subtypes of salivary gland neoplasms and may therefore be useful as biomarkers in diagnostically challenging cases with morphologies partly overlapping with adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma and other salivary gland carcinomas.
16.1.2 Gene Fusion in Carcinoma Ex Pleomorphic Adenoma (CXPA)
Currently, the knowledge about the molecular abnormalities involved in the transformation of a benign pleomorphic adenoma into a carcinoma ex pleomorphic adenoma (CXPA) is still very limited. The malignant component is frequently salivary duct carcinoma but may also a poorly differentiated adenocarcinoma or undifferentiated carcinoma. Molecular studies of small series and single cases of CXPA have shown that they express pleomorphic adenoma-specific gene fusions involving PLAG1 and HMGA2 [5, 54, 76]. In addition, amplification of multiple genes within 12q13–15 (in particular MDM2 and HMGA2-WIF1 gene fusions), TP53 mutation, deletions of 5q23.2–q31.2, gains of 8q12.1 (PLAG1) and 8q22.1–q24.1 (MYC) and amplification of HER2 have been identified as genetic events of importance for malignant transformation of pleomorphic adenoma [107].
16.1.3 Mucoepidermoid Carcinoma
Mucoepidermoid carcinoma (MEC) represents about 5 % of all salivary gland tumours and 20 % of salivary gland malignancies. In a significant number of cases, a recurring t(11;19)(q21;p13) CRTC1-MAML2 (known also as MECT1-MAML2) translocation is present [7, 67, 100]. This alteration results in novel fusion oncogene, in which the CREB-binding domain of the CREB-regulated transcription coactivator CRTC1 (also known as MECT1, TORC1 or WAMTP1) is fused to the transactivation domain of the Notch coactivator MAML2. The fusion protein influences expression of cAMP/CREB (FLT1) and Notch (HES1 and HES5) target genes and is then involved in the transformation of neoplastic cells [7, 71]. Clinical follow-up studies revealed that patients with fusion-positive MECs have a significantly lower risk of local recurrence, metastases and related death compared to fusion-negative patients. When considering tumour-related deaths only, the estimated median survival for fusion-positive patients was greater than 10 years compared to 1.6 years for fusion-negative patients [7]. Seethala and co-workers analysed large series of MEC for presence of the CRTC1-MAML2 translocation by fluorescent in situ hybridisation (FISH) and real-time RT-PCR. Overall, CRTC1-MAML2 translocation was present in 66 % of MEC, whereas all other salivary gland tumours were negative for translocation. Low- or intermediate-grade MEC had a higher frequency of translocation (75 %) than high-grade MEC (46 %) [86]. A novel fusion partner of MAML2 from CRTC family, namely, CRTC3, localised on chromosome 15q26.1, was found to be present in a subset of mucoepidermoid carcinomas [23]. In a relatively recent study, it was shown that CRTC3-MAML2 fusion may be associated with favourable clinicopathological features and patients may be younger than those with CRTC1-MAML2 fusion or those with no detectable gene fusion [65]. When positive for the fusion CRTC1-MAML2 or CRTC3-MAML2, even ‘high-risk’ patients, including those with a higher histological grade or an advanced clinical stage, showed an excellent prognosis [72]. Thus, it was proposed that fusion-positive MECs should be regarded as a distinctive entity separate from fusion-negative cases [39, 72]. Based on a recent array CGH study of genomic imbalances in fusion-positive and fusion-negative MECs, molecular grading of MECs was proposed [39]. The cases can be subdivided in (1) low-grade, fusion-positive tumours with no or few genomic imbalances and favourable prognosis; (2) high-grade, fusion-positive tumours with multiple genomic imbalances (including deletions of the tumour suppressor gene CDKN2A) and unfavourable prognosis; and (3) a heterogeneous group of high-grade, fusion-negative non-MEC adenocarcinomas with multiple genomic imbalances and unfavourable outcome [39]. Molecular analysis can be used to distinguish true MECs, most of which have an excellent prognosis, from fusion-negative MEC-like tumours with a more unfavourable prognosis. There is increasing evidence indicating that the CRTC1-MAML2 fusion is a useful adjunct to histological scoring of mucoepidermoid carcinoma and may lead to development of new clinical guidelines for the management of these patients.
Interestingly, studies have indicated that the CRTC1-MAML2 fusion may not occur exclusively in MEC but also may be found in other types of benign glandular tumours, in particular, in Warthin tumour (WT) of the parotid gland. Putative occurrence of CRTC1-MAML2 fusion transcript has been reported earlier in three case reports of WT, but no histological pictures were provided in these studies [11, 20]. In contrast, other investigators were unable to detect neither the t(11;19) translocation nor CRTC1-MAML2 fusion transcript in any case of WT in large studies [24, 53, 94]. The EWSR1-POU5F1 fusion gene was documented in mucoepidermoid carcinomas of the salivary gland as well as in hidradenoma of the skin, providing further evidence for a genetic link between these two tumour types [61]. This fusion gene appears to be more common in less well-differentiated variants of mucoepidermoid carcinoma, suggesting that it may have prognostic significance [61].
16.1.4 Adenoid Cystic Carcinoma
Adenoid cystic carcinoma (ADCC) is a common malignancy that occurs in both major and minor salivary glands but rarely can be encountered also in exocrine glands in several other anatomical locations, including the breast, sinonasal tract, tracheobronchial tree, cervix and vulva. ADCC of the salivary glands often has a relentless clinical course, characterised by late recurrences and distant metastatic disease. The characteristic molecular feature of ADCCs in all locations appears to be recurrent chromosomal translocation t(6;9)(q22–23;p23–24), which generates a fusion transcript involving the gene for transcription factors MYB and NFIB [68, 76]. MYB is highly expressed in immature cells and plays a key role in proliferation, apoptosis and differentiation. In the translocation t(6;9)(q22–q23;p23–p24), the exon 14 of MYB is fused to the last coding exons of NFIB [76, 99].
The available data indicate that at least 80–90 % of ADCC have MYB activation by gene fusion or other mechanisms leading to overexpression of MYB-NFIB fusion proteins or an apparently normal MYB oncoprotein. In contrast, the MYB-NFIB fusion has not been found in any non-ADCC carcinomas of the head and neck, confirming the high specificity of the MYB-NFIB fusion for ADCC. The fact that MYB activation is found in such a high frequency of ADCCs regardless of the site of tumour origin indicates that the MYB-NFIB fusion is a key oncogenic event and hallmark of AdCC [10, 58, 76, 109]. From a diagnostic point of view, the MYB-NFIB fusion and/or MYB activation may be detected by RT-PCR analysis of fusion transcripts, by FISH analysis using probes for MYB and/or NFIB, or by immunohistochemical staining of MYB-proteins. In addition to being a diagnostic biomarker for ADCC, MYB and its downstream targets are also potential therapeutic targets. The MYB–NFIB t(6;9)(q22–23;p23–24) translocation and MYB protein overexpression have been shown as useful diagnostic marker of adenoid cystic carcinomas and potential prognosticator as MYB translocation-positive cases show tendency towards higher local relapse rate [109].
16.1.5 Mammary Analogue Secretory Carcinoma
In 2010, Skálová and co-workers described a new subtype of salivary gland carcinoma, with strong histological and immunohistochemical resemblance to secretory carcinoma (SC) of the breast, and termed mammary analogue secretory carcinomas (MASCs) of the salivary glands [92]. In addition to the morphologic similarities, MASC and SC of the breast also have important genetic similarities since they both share a t(12;15)(p13;q25) chromosomal translocation [104], resulting in an identical ETV6-NTRK3 gene fusion. The fusion is found in vast majority of MASCs (90 %) and is an important biomarker that may help distinguish MASC from acinic cell carcinoma, low-grade cystadenocarcinoma and other mimics. It should be noted that ETV6-NTRK3 gene fusions are found in several other tumour types, including congenital mesoblastic nephroma, congenital fibrosarcoma and acute myeloid leukaemia [47], indicating that the fusion oncoprotein has transforming activity in a variety of cell types. In context of salivary gland pathology, however, the t(12;15) translocation is rather distinctive being not found in any other salivary gland tumour other than MASC.
16.1.6 Hyalinising Clear Cell Carcinoma
Recently, hyalinising clear cell carcinoma (HCCC) was shown to have t(12;22)(q13;q12) translocations resulting in EWSR1-ATF1 gene fusions [3]. HCCC is a low-grade carcinoma with distinctive clear cell morphology and pattern of hyalinisation often in combination with focal mucinous differentiation. The EWSR1-ATF1 fusion has been found in more than 80 % of HCCC. Interestingly, EWSR1-ATF1 fusions were originally described in conventional clear cell sarcomas (of tendons and aponeurosis) [111] and have recently also been encountered in angiomatoid fibrous histiocytomas as well as in a few cases of soft tissue myoepithelial tumours [2, 25]. Interestingly, the EWSR1-ATF1 gene fusion is not detected in any of the morphological mimics of HCCC, namely, epithelial-myoepithelial carcinoma, low-grade myoepithelial carcinoma or mucoepidermoid carcinoma, demonstrating its usefulness as a diagnostic biomarker for HCCC. Recently, Similar EWSR1 and ATF1 rearrangements have been also identified in clear cell odontogenic carcinoma (CCOC) providing molecular evidence of link between HCCC and CCOC [8]. Moreover, EWSR1 gene rearrangement was recently demonstrated in a subset of high-grade clear cell myoepithelial carcinomas of the salivary gland [95]. The EWSR1 rearranged myoepithelial carcinomas represent distinctive aggressive variant composed predominantly of clear cells with large comedonecroses in the central parts of tumour nests, and they are characterised by poor clinical outcome [95].
16.2 High-Grade Transformation (Dedifferentiation) in Salivary Gland Neoplasms
High-grade transformation or ‘dedifferentiation’ is defined as the abrupt transformation of a well-differentiated tumour into high-grade morphology that lacks the original distinct histological characteristics [26, 63].
16.2.1 Epidemiology and Pathogenesis
High-grade transformation (HGT) has been described in a variety of salivary gland carcinomas. Although the phenomenon is a rare event, it has been reported in acinic cell carcinoma [18, 35, 77, 91, 98], adenoid cystic carcinoma [14, 33, 52, 60, 64, 83, 84], epithelial-myoepithelial carcinoma [1, 45, 81, 85], polymorphous low-grade adenocarcinoma [50, 75, 90], myoepithelial carcinoma [70], low-grade mucoepidermoid carcinoma [64] and mammary analogue secretory carcinoma (MASC) [96]. The molecular genetic mechanisms responsible for the pathway of HGT in salivary gland carcinomas largely still remain to be elucidated. Although data are limited, one or more genes have been documented in high-grade transformation of salivary gland tumours [9, 13, 87, 102].
16.2.2 Histopathology
HGT tumours are composed of conventional carcinomas associated with areas of high-grade morphology, usually either poorly differentiated adenocarcinoma or undifferentiated carcinoma, in which the original line of differentiation is no longer evident. The high-grade component is generally composed of solid nests of anaplastic cells with large vesicular pleomorphic nuclei, prominent nucleoli and abundant cytoplasm (Fig. 16.1). The HGT component frequently exhibits solid growth of tumour cells with greater cytological atypia, higher mitotic frequency and extensive areas of necrosis similar to comedonecrosis (Fig. 16.2). The MIB1 index is consistently higher in the high-grade component (Fig. 16.3). p53 abnormalities have been demonstrated in the transformed component in adenoid cystic carcinomas but is absent in acinic cell carcinoma and MASC. HER2 overexpression and/or gene amplification is considerably exceptional [91].
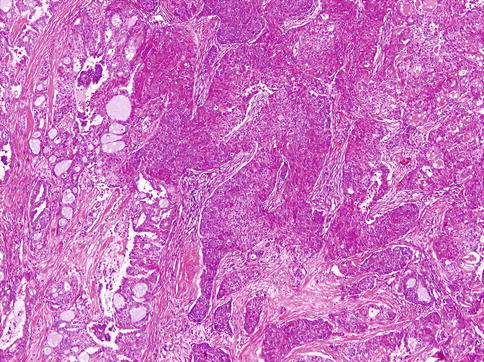
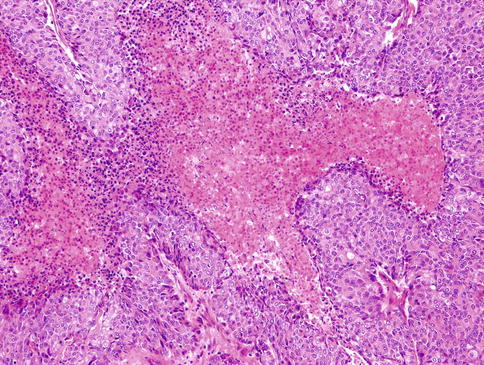
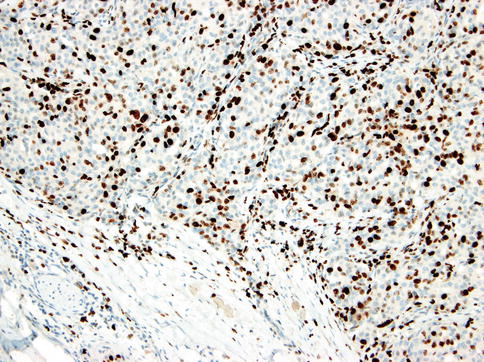

Fig. 16.1
Mammary analogue secretory carcinoma with high-grade transformation: high-grade component is composed of solid nests of anaplastic cells with large vesicular pleomorphic nuclei, prominent nucleoli and abundant cytoplasm

Fig. 16.2
High-grade component frequently exhibits solid growth of tumour cells with greater cytological atypia, higher mitotic frequency and extensive areas of necrosis similar to comedonecrosis

Fig. 16.3
MIB1 index is consistently higher in the high-grade component
16.2.3 Prognosis and Treatment
Salivary gland carcinomas with HGT have been shown to be more aggressive than conventional carcinomas with a poorer prognosis, accompanied by higher local recurrence rate and propensity for cervical lymph node metastasis, suggesting the need for wide resection, neck dissection and possibly also adjuvant chemo/radiotherapy.
16.3 Non-epithelial Tumours, Malignant Lymphoma and Secondary Tumours
Primary salivary soft tissue tumours are rare and account for only a few percent of salivary gland neoplasms. Benign tumours are considerably more common than sarcomas. The majority occur in the parotid gland (more than 85 %) and over 10 % in the submandibular and hence very rarely in the sublingual gland and minor salivary glands [30]. Vascular neoplasms are the most common benign tumour representing approximately 40 % of benign cases, and the vast majority of those (75–80 %) are haemangiomas. Approximately two-thirds of haemangiomas occur in children under 20 years of age [69]. Less common entities are schwannoma, myofibroblastic tumour, nodular fasciitis and lipoma, but also rare cases of, for example, granular cell tumour, osteochondroma, myxoma, solitary fibrous tumour, giant cell lesions, etc., have been described [6, 15, 22, 28, 30, 49, 78, 82, 89, 108]. Although being very rare, most types of sarcomas have been described in the salivary glands, such as the odd cases of, for example, leiomyosarcoma [42] and desmoplastic small round cell tumour [73, 110]. Haemangiopericytoma, malignant schwannoma, fibrosarcoma and malignant fibrous histiocytoma appear to be the most common types [4, 17, 30, 32, 48, 74]. For the histological features of the soft tissue tumours, refer to specific textbooks on the subject.
Primary salivary gland malignant lymphomas are rare, and to be classified as primary, the bulk of the disease should be in the gland and the glandular parenchyma clearly involved [12]. Hodgkin lymphoma is very rare, and several cases described in the literature originated from intraparotid lymph nodes and also the odd case from a pre-existing Warthin tumour [55]. Non-Hodgkin lymphoma (NHL) is more common, albeit a rare primary salivary malignancy, and represents approximately 5 % of all primary extranodal non-Hodgkin lymphomas [27, 56]. The majority of salivary gland NHL are B-cell lymphomas. The extranodal marginal zone B-cell lymphoma (EMZBCL) is likely the most common of the truly primary salivary NHL and is frequently associated with Sjögren syndrome (see also Chap. 2) [12, 40, 43, 57, 79, 80]. For detailed description of the histology and immunoprofile of the different lymphomas, refer to specific textbooks, e.g. the 2008 WHO Classification of Haematopoietic and Lymphoid Tissues [103], and also to educational papers, e.g. like the one by Jaffe in 2009 [38] .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


