Pathogenesis of migraine
The pathogenesis of migraine is imperfectly understood but involves both neuronal and vascular dysfunction (Fig. 26.1). Headache in people with migraine is associated with sensitisation of the pain pathways in both the meninges (peripheral sensitisation) and the central processing of sensory stimuli in the brainstem (central sensitisation). Genetic predisposition is a factor in migraine, and abnormal function of voltage-dependent ion channels in cortical and brainstem structures may provide the basis for increased excitability in the neuronal circuits that are responsible for the headache.
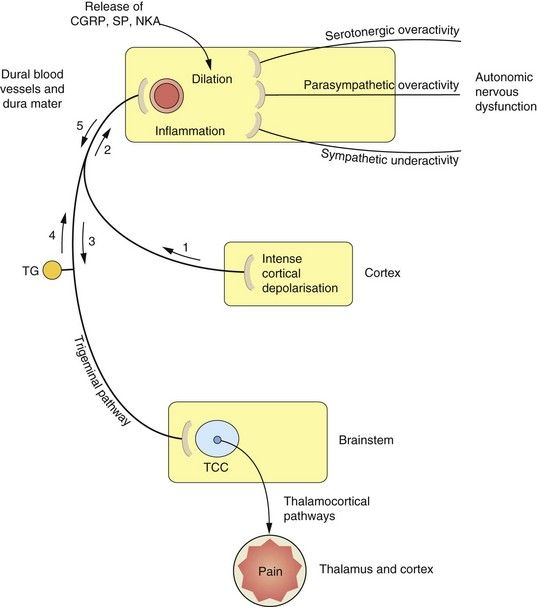
Fig. 26.1 Proposed mechanisms associated with the processes of migraine.
The sequence and nature of neurogenic and vascular involvement in migraine are vigorously debated and may vary in different kinds of migraine-type headache. One scenario is that the trigger is a wave of cortical depression (1) which sensitizes and stimulates the trigeminal pathway in either direction (2, 3). This results in stimulation of the trigeminocervical complex (TCC) (3) in the brainstem, and onward stimulation of the thalamus and other areas can cause pain and nausea. Other reflex pathways from the brainstem via the superior salivatory nucleus to the dural blood vessels, which result in vasodilation, can also be activated at this stage. The stimulation of trigeminal nerves innervating the dural blood vessels (2, 4) results in the release of mediators such as calcitonin gene-related peptide (CGRP), substance P (SP) and neurokinin A (NKA), which cause vasodilation and participate in inflammation. CGRP and possibly other mediators are able to stimulate nociceptors in the trigeminal nerve endings, resulting in further activation of the pathways to the TCC and thalamus (5) and consequently further pain. The control of vascular tone in the dural blood vessels is complex, with sympathetic, parasympathetic and serotonergic systems contributing to the migraine process. Vasoconstrictor innervation of these vessels is by sympathetic nerves and vasodilation occurs by parasympathetic innervation. Serotonergic pathways produce vasoconstriction by stimulation of 5-HT1B receptors and vasodilation via 5-HT2 receptors. TG, trigeminal ganglion.
An aura precedes a migraine attack in 15% of cases with transient visual symptoms (such as flashing or jagged lights) or sensory symptoms. Visual symptoms are probably caused by an initial intense but brief neuronal excitation producing cortical hyperaemia. This is followed by a slowly propagated wave of cortical depolarisation that transiently depresses spontaneous and evoked neuronal activity (cortical spreading depression). The depressant wave is associated with vasoconstriction, which may produce focal neurological symptoms and signs. The majority of migraineurs who do not experience an aura appear to have ‘silent’ cortical spreading depression and this suppression of electrical activity may therefore be the trigger for the migraine headache.
The hallmark of the migraine process is activation of the trigeminal nerve pathway (Fig. 26.1). Increased dopaminergic and glutamatergic neurotransmission, with reduced serotonergic neurotransmission, are all thought to contribute to this activation.
The migraine process involves vasodilation and inflammation of the dural blood vessels and dura mater (neurogenic inflammation). This results from activation of afferents in the trigeminal pathway from the trigeminocervical complex in the brainstem to the dural blood vessels. The triggers that activate the pathway are unknown, but are probably chemical. Activation of the pathway releases vasoactive peptides such as calcitonin gene-related peptide (CGRP) and substance P from the perivascular axons in the dura mater. As a result, the local concentrations of cytokines, serotonin, histamine and nitric oxide increase, producing vasodilation and activating endothelial cells, mast cells and platelets. Mediators released from these cells further contribute to neurogenic inflammation. Sensory nociceptors on the trigeminal nerves in the dura are stimulated, and the nociceptive impulses are relayed from the dura through the trigeminocervical complex in the brainstem, from where they are transmitted to the thalamus. Thalamic stimulation produces pain, nausea and vomiting. Monoaminergic pathways in the trigeminocervical complex modulate the nociceptive pain (Ch. 19) via afferent nerves to intra- and extracranial blood vessels. There are also reflex parasympathetic connections back to the dural vessels that potentiate vasodilation (Fig. 26.1). The trigeminocervical complex is rich in serotonin 5-HT1B/1D receptors, and their stimulation (particularly 5-HT1B receptors) inhibits neurotransmission to the thalamus.
Drugs for migraine
Specific drugs for the acute migraine attack
Triptans
Mechanisms of action and effects: The triptans are serotonin 5-HT1B/1D receptor agonists, which may alter the pathophysiology of migraine (Fig. 26.2) by:
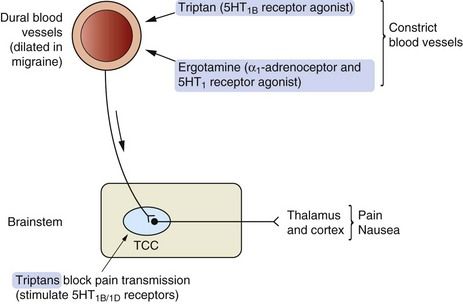
Fig. 26.2 Possible mechanisms of action of 5-HT receptor agonists in migraine.
The trigeminocervical complex (TCC) is rich in 5-HT1B/1D receptors and stimulation of these receptors by so-called second-generation triptans inhibits neurotransmission to the thalamus and cortex. Triptans also cause vasoconstriction acting at 5-HT1B receptors on blood vessels.
Sumatriptan does not easily cross the blood–brain barrier since it is water-soluble, but this barrier may be impaired during a migraine attack. Naratriptan, zolmitriptan and other ‘second-generation’ triptans penetrate the blood–brain barrier more readily. These drugs directly inhibit excitability of the trigeminocervical complex in the brainstem and relieve both the pain and the nausea associated with migraine.
Pharmacokinetics: Absorption of sumatriptan from the gut is rapid but erratic, whereas second-generation triptans such as naratriptan and zolmitriptan have better absorption. Effective plasma concentrations of these drugs are usually reached within 30 min of oral administration. Sumatriptan and zolmitriptan undergo first-pass metabolism by monoamine oxidase A (MAO-A) and have a low oral bioavailability. Subcutaneous injection or administration by nasal spray avoid first-pass metabolism with these drugs and relieves symptoms within 15 min. These routes can be more effective if the headache is accompanied by nausea, because gastric stasis often delays oral drug absorption during a migraine attack. Naratriptan is not a substrate for MAO-A, which gives it high oral bioavailability. Elimination of most triptans is partially by hepatic metabolism and partially by renal excretion. Most triptans have short half-lives of between 2 and 6 h, but the half-life of frovatriptan is longer, at 26 h.
Unwanted effects: The frequency and intensity of unwanted effects is highest after subcutaneous use of sumatriptan:
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree













