Fig. 6.1.
(A) Staphylococcus aureus in gram-stained smear of purulent exudate, (B) Botryomycotic abscess (H&E).
Special Stains
♦
Staphylococci are 0.5–1.0 μm gram-positive spherical to “kidney bean”-shaped cocci with a tendency to form clusters
♦
Brown–Brenn is generally the best tissue gram stain for gram-positive bacteria
♦
Brown–Hopps is generally the best tissue gram stain for gram-negative bacteria
♦
MacCallum–Goodpasture, another modified tissue gram stain, can also be used to determine the gram reaction of bacteria
Differential Diagnosis
♦
Impetigo – the differential includes herpes simplex or varicella zoster
♦
Abscess – the differential includes other nonanaerobic bacteria and various anaerobic bacteria
♦
Cellulitis and fasciitis – the differential includes Streptococcus pyogenes, anaerobes, and other bacteria
♦
Streptococcus pyogenes is an additional cause of toxic shock syndrome
♦
Botryomycosis – the differential includes:
Actinomycotic granules
Deep mycotic infections
Diagnostic Techniques
♦
Culture or other means of demonstrating microorganism may be required for definitive diagnosis
♦
Antimicrobial susceptibility testing aids in selecting antibiotics for treatment
Staphylococcus epidermidis and Other Coagulase-Negative Staphylococci (CoNS)
♦
Identical to S. aureus with respect to their gram-stain features and microscopic appearances. These catalase-positive staphylococci differ from S. aureus by their negative coagulase reaction
Clinical
♦
CoNS inhabit normal human skin and mucosal surfaces
♦
In the past, the less virulent coagulase-negative staphylococci were generally regarded as insignificant contaminants. Through the years, however, it has become clear that these bacteria are important pathogens, capable of producing human disease
♦
In general, S. epidermidis is the most prevalent of CoNS in infections
♦
Nosocomial bacteremia – CoNS are the most common cause of nosocomial bacteremia ; however:
Typically, 1–3% of blood cultures are contaminated with CoNS
Approximately 75–90% of CoNS isolated from blood cultures are contaminants
♦
Endocarditis of native and prosthetic valves
CoNS cause approximately 5–8% of all cases of bacterial endocarditis; CoNS are not common causes of native valve endocarditis
In contrast, CoNS are the most common cause of prosthetic valve endocarditis (approximately 40% of cases)
♦
Intravenous catheter infections – S. epidermidis is the single most common cause of these infections
♦
Cerebrospinal fluid (CSF) shunt infections – S. epidermidis is the most common microorganism involved
♦
Prosthetic joint infections (e.g., of the hip and knee) can involve CoNS
♦
Urinary tract infections in young, sexually active women may be due to CoNS
S. saprophyticus is a common cause of these infections
Diagnostic Techniques (Fig. 6.2)
♦
Microscopic and special stain features of CoNS are i ndistinguishable from those of S. aureus
♦
Identification of isolates in the microbiology laboratory is based on relatively simple biochemical tests
♦
Molecular-based assays such as PCR and PNA-FISH probes are also becoming widely available and can often be used directly on positive specimens such as blood culture bottles
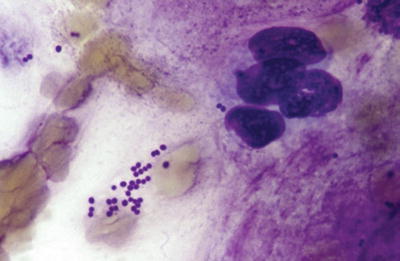
Fig. 6.2.
Coagulase-negative Staphylococcus species in sputum specimen (Diff-Quik).
Streptococcus pneumoniae (Pneumococcus)
Clinical
♦
This catalase-negative gram-positive coccus colonizes the nasopharynx of about:
5–10% of healthy adults
20–40% of healthy children
♦
It is a common cause of community-acquired pneumonia in adults
♦
S. pneumoniae is the most common cause of meningitis in adults, except during outbreaks of Neisseria meningitidis
♦
S. pneumoniae is the most frequent cause of meningitis in children in countries where vaccination for Haemophilus influenzae type B is common
♦
This organism is the most prevalent pathogen in otitis media in children (40–50% of cases), with the second most prevalent being nontypeable H. influenzae
♦
S. pneumoniae also is the most common cause of otitis media in adults
♦
In a number of studies, S. pneumoniae has usually ranked as the most common cause of acute paranasal sinusitis, second only to nontypeable H. influenzae; acute paranasal sinusitis is often preceded by a viral infection (e.g., common cold)
Macroscopic
♦
Lobar pneumonia – most cases are caused by S. pneumoniae; less frequent causes include Klebsiella pneumoniae, staphylococci, streptococci, H. influenzae, and Legionella spp
♦
The classic stages follow:
Congestion is usually seen in the first 24 h
Red hepatization – the lung appears red and firm, with a liver-like consolidation (due to abundant neutrophils and erythrocytes)
Gray hepatization – there is lytic destruction of erythrocytes with persistent fibrinosuppurative exudate
Resolution – in this stage, the exudate is destroyed and cleared; the lung is restored to normal
Complications – empyema, lung abscess, and pericarditis may occur
♦
Meningitis
The CSF is cloudy or purulent (abundant neutrophils and bacteria)
Leptomeningeal exudate is often the thickest over the cerebral convexities near the sagittal sinus
Meningeal vessels are engorged and prominent
The underlying brain is swollen
Microscopic (Fig. 6.3)
♦
An acute neutrophilic host response occurs
♦
Polysaccharide capsule (~90 antigenic serotypes), which prevents pneumococci from being phagocytosed, is often suggested by the halolike area around the cocci
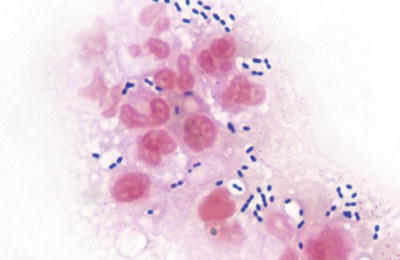
Fig. 6.3.
Streptococcus pneumoniae in sputum (gram).
Special Stains
♦
Gram stain – the organisms appear as slightly elongated, “lancet,” or “football”-shaped gram-positive cocci, usually in pairs in clinical materials
♦
A halolike appearance of capsule against the background is often prominent
Differential Diagnosis
♦
Enterococcus species, although not common in respiratory infections or meningitis, can be morphologically similar to S. pneumoniae
♦
Other genera and species may form pairs of cocci (including staphylococci and other streptococci), but most are not “lancet” or “football” shaped
Diagnostic Techniques
♦
Good-quality sputum for culture is essential (microbiology laboratories reject poor quality “sputa” based on established criteria)
♦
Blood cultures are indicated in patients clinically suspected of having bacterial pneumonia
Approximately 25% of patients with pneumococcal pneumonia have positive blood cultures
♦
Urine assays for pneumococcal polysaccharide are positive in two-third of patients with pneumococcal pneumonia but also positive in up to 15% of patients with nonpneumococcal pneumonia and may be positive in healthy children with nasopharyngeal colonization
Streptococcus pyogenes (Group A Streptococcus)
Group A streptococci are catalase-negative, gram-positive, spherical to ovoid cocci that occur in pairs or chains
Also one of the most important bacterial pathogens of humans, it colonizes throats of 15–20% of asymptomatic school children; the carriage rate in adults is lower
Clinical
♦
Pharyngitis and tonsillitis
S. pyogenes is the most common bacterial cause of these infections
One of the potential postinfectious, nonsuppurative complications of S. pyogenes pharyngitis is rheumatic fever
Immune complex glomerulonephritis (poststreptococcal glomerulonephritis), in contrast to rheumatic fever, is a postinfectious nonsuppurative sequela of both S. pyogenes pharyngitis and impetigo (pyoderma; see below)
♦
Scarlet fever
This is a complication involving a S. pyogenes strain lysogenized by a bacteriophage that produces a pyrogenic exotoxin
It is usually associated with pharyngitis and tonsillitis
It is most common between the ages of 3 and 15 years
There is a prominent punctate, erythematous rash that is most abundant over the trunk and inner aspects of the arms or legs
The face is involved but the area around the mouth is unaffected (circumoral pallor)
♦
Erysipelas
This is an acute inflammatory process involving the skin due to S. pyogenes (and occasionally group C strep)
It mostly occurs in infants and in adults over 30
It is characterized by rapidly spreading, cutaneous swelling, and a rash involving the face in a “butterfly” distribution
♦
Impetigo (pyoderma)
This is a focal, purulent infection of the skin caused by S. pyogenes which follows colonization after exposure to fomites or contact with an infected person
•
A minor scratch, insect bite, or another type of break in the skin introduces the organism into the skin and subcutaneous tissue
Groups C and G streptococci have also been implicated
S. aureus is also commonly encountered in impetigo
The infection involves the superficial layer of the epidermis with production of pus-filled vesicles that crust over; with scratching, they readily spread
♦
Streptococcal cellulitis
This takes the form of an acute, spreading inflammation of the skin and subcutaneous tissues
It results from infection of burns, wounds, or surgical incisions
Predisposing factors include intravenous drug injection and impaired lymphatic drainage from the upper (e.g., postmastectomy) or lower extremities
♦
Necrotizing fasciitis (streptococcal gangrene, or “hospital gangrene”)
It was described in 1924 by Frank Meleney
Predisposing factors include:
•
Trauma or appendicitis in young people
•
Diabetes, congestive heart failure, or surgical trauma in older people
The infection involves the deeper subcutaneous tissue and fascia
Extensive/rapidly spreading necrosis and gangrene of the skin and underlying structures are characteristic features
The mortality of this condition is high: 20–70%
Virulent S. pyogenes sometimes called “flesh-eating bacteria ” by the media
♦
Nonsuppurative poststreptococcal sequelae (described in other chapters)
Acute rheumatic fever
•
This is characterized by nonsuppurative inflammatory lesions involving primarily the heart, joints, and subcutaneous tissues
•
It follows group A streptococcal upper respiratory infections
Poststreptococcal glomerulonephritis
•
This is an acute inflammatory disorder of the renal glomerulus characterized by edema, hypertension, hematuria, and proteinuria
•
It is a delayed sequela of pharyngeal or cutaneous infection with certain “nephritogenic” strains
Microscopic (Fig. 6.4)
♦
Scarlet fever
The dermis is edematous and hyperemic with scant perivascular lymphocytes and other mononuclear cells
Inflammation of the epidermis is followed by hyperkeratosis and desquamation of the skin
♦
Erysipelas
The epidermis shows spongiosis
There is acute edematous, neutrophilic inflammation in the dermis; it is prominent around vessels and adnexa and extends into subcutaneous tissue
♦
Impetigo (pyoderma) due to S. pyogenes
It resembles impetigo caused by S. aureus
However, abscess formation is less common with S. pyogenes than with S. aureus
♦
Streptococcal cellulitis
This is characterized by diffuse interstitial, neutrophilic infiltrates within cellular connective tissue
It involves minimal damage to host tissue
♦
Necrotizing fasciitis (streptococcal gangrene)
This is characterized by hemorrhage, edema, and necrosis plus mixed inflammatory cell inflammatory infiltrate that dissects along fascial planes
It is important to note that bacteria are not typically seen in the damaged tissue; the condition is toxin mediated
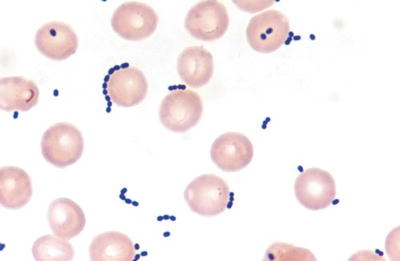
Fig. 6.4.
Streptococcus species in positive blood culture (gram).
Special Stains
♦
Streptococci may be difficult to see in H&E-stained sections
♦
Brown–Brenn is the preferred tissue gram stain
♦
These streptococci are spherical or ovoid gram-positive cocci, often in short chains in histologic sections; the finding of >10 cocci per chain aids in recognizing streptococci in stained smears of body fluids, exudates, and cultures
Differential Diagnosis
♦
Staphylococci cause similar infections, but tend to form clusters and almost never form long chains
♦
Other bacteria including enterococci and anaerobic cocci share similar microscopic features
Some anaerobes cause similar infections
Streptococcus agalactiae (Group B Streptococcus)
♦
Group B streptococci are catalase-negative, gram-positive cocci that occur in pairs or chains
♦
This species is an important bacterial pathogen of neonates and infants under 3 months, pregnant women, and also older adults
♦
It is an asymptomatic colonizer of the lower gastrointestinal tract and genitourinary tract (20–40% of adult women)
♦
Approximately 60% of infants born to colonized mothers become colonized with the mother’s S. agalactiae
Clinical
♦
Early-onset neonatal disease
Occurs during the first week of life. The organism is acquired in utero or at birth
It is characterized by sepsis, pneumonia, and meningitis
♦
Late-onset neonatal disease (older infants)
This occurs between 1 week and 3 months of life
It is acquired from the mother or another infant
The predominant manifestations are pneumonia with meningitis
♦
In pregnant and postpartum women, S. agalactiae can cause urinary tract infections and endometritis
♦
In addition, S. agalactiae is an emerging cause of morbidity and mortality (15% to >30%) in men and nonpregnant women
Generally, in older individuals. Diabetes mellitus and underlying liver disease (e.g., secondary to alcohol) are the most common predisposing factors
The most common manifestations are bacteremia, pneumonia, bone, and soft tissue infections
Microscopic
♦
Congenital pneumonia (due to contamination of amniotic fluid after premature rupture of membranes or prolonged labor) which often has a lobar distribution
It is characterized by neutrophilic inflammation; it lacks fibrin (amniotic fluid is fibrinolytic)
Squamous debris and other elements of meconium are present
♦
Perinatally acquired pneumonia due to S. agalactiae
Hyaline membranes and neutrophilic infiltrates are often present
Sections show the presence of large numbers of gram-positive cocci in hyaline membranes
♦
Purulent meningitis
Meningitis due to S. agalactiae resembles meningitis caused by other bacteria
Beyond the newborn period, it is rare in infants or in older children
Special Stains
♦
Brown–Brenn is the preferred tissue gram stain
Differential Diagnosis
♦
The distinction from noninfectious hyaline membrane disease can be made by demonstrating bacteria in the hyaline membranes plus a more pronounced neutrophilic inflammation
♦
S. agalactiae and Escherichia coli are tied for first place as the most common bacterial causes of neonatal meningitis
Their microscopic features differ
Enterococcus Species
♦
Previously included with the group D streptococci, these catalase-negative, gram-positive cocci normally inhabit the gastrointestinal tracts of humans and other animals
♦
The most commonly encountered Enterococcus species in infections are E. faecalis (~80–90%) and E. faecium (~10–15% or more of enterococcal isolates)
Clinical
♦
Patients at increased risk of infection with enterococci include those hospitalized for long periods of time and those being treated with broad-spectrum antibiotics
These bacteria are inherently resistant to many commonly used antibiotics (e.g., oxacillin, cephalosporins, and other β-lactam agents)
They also may acquire resistance genes (e.g., aminoglycosides, vancomycin)
♦
They cause approximately 10% of nosocomial infections
♦
Enterococcal urinary tract infections are common in patients with urinary catheters
♦
Wound infections (e.g., intra-abdominal); many such infections are polymicrobial and the significance of the Enterococcus species in these infections is not always clear
♦
Bacteremia due to enterococci may be highly clinically significant, especially in patients with intravascular catheters
♦
Bacterial endocarditis; Enterococcus species cause ~15% of cases
♦
Pneumonia (well-documented but unusual)
♦
Meningitis (a rare cause)
Microscopic (Fig. 6.5)
♦
Although enterococci have relatively low virulence, acute inflammation is associated with these infections
♦
These bacteria are gram-positive cocci that occur in singles, pairs, and chains of varying lengths
♦
They can be encapsulated
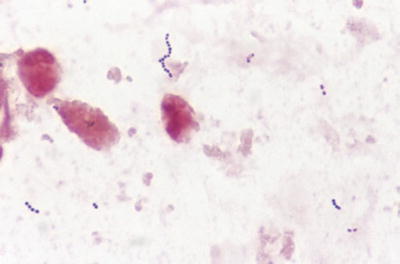
Fig. 6.5.
Enterococcus faecium pneumonia (bronchoalveolar lavage specimen [gram]).
Differential Diagnosis
♦
They frequently are indistinguishable from Streptococcus pneumoniae on direct microscopic examination
♦
Though “lancet-shaped” cocci in pairs are typically formed by S. pneumoniae, it can also form chains
♦
Enterococci can vary from spherical to ovoid or somewhat “lancet shaped”
Alpha-Hemolytic Streptococci (Viridans Streptococci)
♦
“Viridans” is a descriptive name, not a taxonomic name, for Streptococcus species other than S. pneumoniae that produce partial hemolysis of erythrocytes in blood agar
Clinical
♦
Certain oral species, particularly Streptococcus mutans, play a significant role in dental plaque formation and dental caries
♦
Other types of clinical settings from which alpha-hemolytic streptococci (e.g., S. sanguis, S. constellatus, S. intermedius, and/or others) are isolated include bacteremia, infective endocarditis, and aspiration pneumonia
♦
The members of the Streptococcus anginosus group (formerly Streptococcus milleri group) which includes S. constellatus, S. intermedius, and S. anginosus have a propensity to form abscesses in soft tissues and solid organs
Gram-Positive Bacilli
Bacillus anthracis (Etiologic Agent of Anthrax)
♦
B. anthracis is a sporeforming (subterminal location), gram-positive rod, which grows aerobically and produces catalase
It differs from other Bacillus species encountered in clinical microbiology laboratories by its lack of hemolysis on blood agar, inability to hydrolyze gelatin or ferment salicin, and its absence of motility
♦
Historically, anthrax has been a disease of animals having contact with soil contaminated with spores and herbivorous animal-associated disease of humans (e.g., “woolsorter’s disease”)
Although still endemic in many countries, animal-associated disease of humans became uncommon in the USA as a result of vaccinating animals and preventive measures applied to the processing of materials from animals (e.g., wool and hides)
B. anthracis has also been used as an agent of biological warfare by a number of countries
In 1992, weaponization of B. anthracis through the years was admitted by the former Soviet Union along with information that in 1979 an accidental release of B. anthracis spores from a Soviet military laboratory in Sverdlovsk resulted in at least 77 cases of inhalational anthrax with 66 mortalities
Then in 2001 in the USA, both cutaneous and inhalational forms of anthrax were acquired in people exposed to B. anthracis spores that had been sent in contaminated letters as a deliberate act of bioterrorism
The major virulence factors of B. anthracis are the antiphagocytic polypeptide capsule (poly-d-glutamic acid) and anthrax toxins
Anthrax toxins contain three protein components, the protective antigen (PA), edema factor (EF), and lethal factor (LF)
•
The proteins are not toxic by themselves
•
PA + EF forms edema toxin, which has adenylate cyclase activity and is responsible for the major fluid accumulation in anthrax
•
PA + LF forms lethal toxin, which is the major virulence factor
Accordingly, the zinc metalloprotease activity of the lethal toxin causes macrophages to release tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and other cytokines
Lethal toxin, depending upon its concentration, also blocks macromolecular synthesis in macrophages and causes macrophages to lyse
Clinical
♦
Cutaneous
About 95% or more of naturally occurring cases are cutaneous
The incubation period is 1–12 days
The lesions usually occur on exposed skin of the head, neck, or an upper extremity
♦
Inhalational (woolsorter’s disease)
Inhalational anthrax may develop 1–5 days after inhaling spores
•
However, there may be a long latent period (>1 month) with spores in the nasal passages or in the lower respiratory tract that subsequently are phagocytized by macrophages and transported to mediastinal lymph nodes
The illness begins with a “flu-like” onset with fever, myalgias, and a nonproductive cough
There is a left shifted neutrophilia
Severe respiratory distress begins with a sudden onset
Mediastinal widening is a characteristic radiographic feature
Without early recognition and treatment, shock followed by death within 1–2 days occurs in most patients
♦
Gastrointestinal
This is rare; most reported cases are from Africa and Southeast Asia
It is acquired through ingestion of B. anthracis spores (e.g., in contaminated undercooked meat)
Symptoms include nausea, vomiting, anorexia, fever, abdominal pain, and severe bloody diarrhea
Macroscopic
♦
The hallmark is hemorrhage and edema
♦
Cutaneous
It begins as a papule that is usually pruritic resembling a bug or spider bite
By 48 h, the cutaneous lesion becomes a pruritic vesicle surrounded by edematous, blue-black tissue
The vesicle subsequently becomes necrotic and hemorrhagic, producing the classic “black eschar” that is painless and usually self-limiting. In contrast, spider bites are usually painful
♦
Inhalational
Hemorrhagic lymphadenitis and mediastinitis are characteristic features
Manifestations of hematogenous spread occur, especially hemorrhagic meningitis (i.e., “cardinals cap”)
♦
Gastrointestinal
A localized oropharyngeal lesion may occur
•
This is less common than intestinal involvement
This may occur as a severe systemic disease with a localized necrotizing lesion in the terminal ileum or cecum containing many organisms
Microscopic
♦
Features include hemorrhage, edema, necrosis, fibrin deposition, increased neutrophils, and profound bacillemia
♦
Lymphoid necrosis (with sinus histiocytosis and hemorrhage in lymph nodes) is a prominent finding
♦
Necrotizing vasculitis in small cerebral vessels is a prominent feature of hematogenous spread
Special Stains
♦
B. anthracis is a gram-positive, large “box-car”-shaped bacillus
It tends to form short chains of vegetative cells in tissue and long chains in culture
♦
Spores are not seen in histologic sections or in smears of fresh clinical specimens
Differential Diagnosis
♦
The differential includes staphylococcal cellulitis, tularemia, plague, cat-scratch disease, rat bite fever, and sporotrichosis
♦
Taking a good history can be the most important step in making a correct diagnosis
Diagnostic Techniques
♦
Specimens to be collected from suspected anthrax patients include:
Cutaneous
•
Collect vesicular fluid with swabs for culture and direct smear preparation, full-thickness punch biopsy fixed in 10% neutral-buffered formalin
•
If present, biopsies of vesicle and eschar should also be taken
Intestinal
•
If an adequate history suggests intestinal anthrax, collect feces and blood for culture
•
If there is an autopsy, collect the following for smears and cultures: blood; hemorrhagic fluids from the nose, mouth, or anus; and aspirates from the spleen and/or lymph nodes
Inhalational
•
If the patient is ill and an adequate patient history suggests it, blood for culture should be collected
•
At autopsy, the same materials as described for intestinal anthrax should be collected
♦
The organism grows well on sheep blood agar (“producing Medusa head colonies”) and other media
♦
B. anthracis is one of the Category A agents of bioterrorism
These are agents that pose the greatest threat relative to their infectious nature, ease of spread, or high mortality (e.g., Bacillus anthracis, Clostridium botulinum [botulinum toxins], Francisella tularensis, Brucella species [see text], Yersinia pestis, variola major [smallpox], filoviruses [Marburg virus, Ebola virus], and arenaviruses [Lassa virus, Machupo virus])
♦
In a healthcare setting, laboratory procedures to be performed with B. anthracis must be done in a facility with a biosafety level of at least 2. Culture isolates suspected of being B. anthracis should only be manipulated in a biosafety cabinet
Activities that are at high risk for creating aerosols with this organism should be done in a biosafety level 3 laboratory
♦
If anthrax is suspected in a clinical laboratory or sentinel laboratory, local and state public health authorities must be notified immediately
♦
The Laboratory Response Network toll-free HelpDesk ((866) 576–5227) is available for subject matter experts if discussions on individual organisms are needed
♦
Another excellent source of laboratory information relative to these types of agents is the American Society for Microbiology website (http://www.asm.org/index.php/guidelines/sentinel-guidelines)
Bacillus cereus
♦
This is a sporeforming, gram-positive rod, which grows aerobically and produces catalase
♦
Unlike B. anthracis, B. cereus produces hemolysis on sheep blood agar and is motile
♦
B. cereus is ubiquitous in nature and the hospital environment
♦
Other Bacillus species such as B. subtilis and B. sphaericus are common laboratory contaminants
Clinical
♦
Gastrointestinal (GI) illness
One form is the toxin-induced emetic form (associated with consumption of contaminated reheated rice)
•
It has a short incubation period of 1–6 h similar to staphylococcal food poisoning
The second form of GI illness caused by B. cereus is diarrheagenic food poisoning
•
It has an incubation period of 10–12 h similar to Clostridium perfringens food-borne illness
♦
Non-GI Infections include the following:
Local (ocular, trauma, burn, and wound infections)
Bacteremia/septicemia (intravenous drug use, indwelling catheters, hemodialysis)
Central nervous system (shunt or trauma associated)
Respiratory
Endocarditis
Macroscopic
♦
Cutaneous infections may begin as a single necrotic bulla
♦
Reports of bacillus central nervous system infections have described brain abscess, subarachnoid hemorrhage, and necrotic lesions at the gray-white junctions
Microscopic (Fig. 6.6)
♦
Vasculitis, coagulative necrosis, and hemorrhage may be seen
♦
Large clusters of large bacilli may be found in necrotic areas
♦
There may be little to no inflammatory response in neutropenic patients

Fig. 6.6.
Bacillus species (microbiologic culture [gram]).
Special Stains
♦
Brown–Brenn gram stain may reveal sporeforming gram-positive or gram-variable bacilli
♦
B. cereus is usually PAS+ after diastase digestion
Differential Diagnosis
The differential diagnosis includes the following:
♦
Clostridium species
♦
Bacillus anthracis
Diagnostic Techniques
♦
Culture is required for definitive diagnosis
Corynebacterium diphtheriae
♦
Diphtheria is caused by C. diphtheriae, a pleomorphic gram-positive rod with swollen ends that forms pallisading arrangements sometimes referred to as “picket fence” arrangements or “Chinese letters”
Clinical
♦
There is respiratory spread
♦
C. diphtheriae proliferates at the site of attachment (e.g., oropharyngeal pseudomembrane)
♦
Ulcerative cutaneous lesions also may be primary sites of infection
♦
A phage-encoded exotoxin damages the heart and peripheral nerves
Macroscopic
♦
The diphtheritic pseudomembrane consists of a thick, gray or black, tenacious exudate
It adheres to the submucosa
Bleeding and asphyxiation may result if the pseudomembrane is removed or sloughed away
♦
Skin ulcers also may be covered with gray pseudomembranes
Microscopic
♦
The pseudomembrane contains fibrin, neutrophils, and necrotic debris
♦
Exotoxin damage to the heart is manifested by fatty myocardial change with isolated myofiber necrosis
♦
Exotoxin-induced neuritis is manifested by degeneration of myelin sheaths
Special Stains
♦
Loeffler methylene blue stain, done on smears prepared from overnight growth on Loeffler medium, can aid in providing a preliminary or presumptive diagnosis by revealing diphtheroid forms with metachromatic granules
♦
Immunofluorescent staining of 4 h cultures is also useful
Differential Diagnosis
♦
The differential includes infectious mononucleosis, streptococcal or viral pharyngitis and tonsillitis, Vincent angina, and acute epiglottitis
♦
Definitive diagnosis requires microbiologic culture
Swab specimens are inoculated onto sheep blood agar and/or colistin–nalidixic acid agar, along with cystinetellurite blood agar or modified Tinsdale agar for the isolation of C. diphtheriae
Colonies resembling those of C. diphtheriae must be identified by biochemical testing (nucleic acid sequencing is used in some laboratories)
Toxigenicity testing is also required
•
This can be done using a modified Elek immunoprecipitation–immunodiffusion test or by alternative immuno methods
•
PCR methods have also been developed that detect gene sequences of toxigenic C. diphtheriae
Corynebacterium jeikeium (Formerly Group “JK” Bacilli)
♦
C. jeikeium shows clinically important resistance to most antibiotics except vancomycin. Most infections by this species are nosocomial
Clinical
♦
Infections caused by C. jeikeium and other Corynebacterium species include:
Intravenous catheter-related bloodstream infections
Skin and soft tissue infections
Pneumonia
Prosthetic valve and native valve endocarditis
Cerebral spinal fluid shunt infections and meningitis
Continuous ambulatory peritoneal dialysis-related peritonitis
Microscopic (Fig. 6.7)
♦
C. jeikeium occurs as gram-positive pleomorphic rods; in pallisading arrangements sometimes referred to as “Chinese letters,” “birds in flight,” and “picket fence”
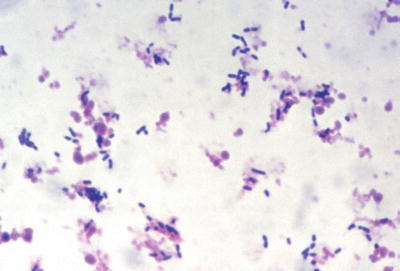
Fig. 6.7.
Corynebacterium jeikeium, in positive blood culture (gram).
Differential Diagnosis
♦
Definitive diagnosis requires microbiologic culture and identification of the organism
Listeria monocytogenes
♦
L. monocytogenes is a small, coccobacillary gram-positive, nonsporeforming rod that is capable of growth at 4 °C, motile at room temperature, poorly motile or nonmotile at 37 °C, weakly β-hemolytic on blood agar, and catalase positive
♦
It can be isolated from soil, water, sewage, decaying vegetation, the fecal microbiota of various animals, and feces of about 1–5% of asymptomatic humans
♦
It is a facultative intracellular pathogen that can infect many different domesticated and nondomesticated animals and humans
Clinical
♦
It causes food-borne illness, both sporadically and in large outbreaks associated with contaminated foods (e.g., contaminated dairy products, undercooked meats, poultry, unwashed vegetables, and cabbage)
♦
The organism causes mild illness (e.g., gastroenteritis and flu-like illness) in otherwise healthy adults
♦
Pregnant women, usually associated with their decline in cell-mediated immunity during the third trimester, are at increased risk of developing listerial bacteremia clinically manifested as an acute febrile illness usually accompanied by chills, headache, myalgias, arthralgias, and backache
In contrast to others who are prone to develop listeriosis, meningitis is rare in pregnant women
♦
The organism can spread transplacentally resulting in amnionitis and life-threatening in utero infection
This can result in spontaneous abortion, premature birth, or death may occur within hours due to a disseminated form of listeriosis (i.e., granulomatous infantiseptica with abscesses of many organs)
♦
Listeria is an important cause of clinically significant bacteremia and meningitis in immunocompromised patients (e.g., bone marrow and organ transplant recipients; patients with AIDS)
Microscopic and Special Stains
♦
In acute meningitis, the findings are nonspecific and similar to those seen in pyogenic meningitis caused by Neisseria meningitidis or Haemophilus influenzae
♦
The presence of small gram-positive rods in cerebrospinal fluid can be diagnostic, though L. monocytogenes may be confused with H. influenzae, if it is overdecolorized. It may also appear as pairs of cocci suggesting S. pneumoniae, or it may resemble Propionibacterium or Corynebacterium and mistakenly thought to be a skin contaminant
♦
Placentitis is characterized by acute inflammation and abscess formation
In suspected prenatal infection, the placenta and fetal membranes should be examined and cultured
The mother’s reproductive tract also should be cultured
Nocardia Species (Nocardiosis)
♦
Nocardiosis is a disseminated or localized infection caused by obligately aerobic, gram-positive, branching, filamentous rods of the genus Nocardia
Classification
♦
The organism is classified with the “aerobic nocardioform” subgroup of the “aerobic actinomycetes,” taxonomically in the class Actinobacteria, order Actinomycetales
♦
This is a large and diverse group of bacteria, many of which are only distantly related phylogenetically
♦
Genera in this subgroup include Nocardia, Rhodococcus, Gordona, Tuskamurella, Dietzia, and Mycobacterium
♦
In culture, these bacteria produce long, branching filaments that tend to break up into rod-shaped and coccoid forms
Epidemiology and Clinical Manifestations
♦
Nocardia species are ubiquitous in soil, organic materials, and water; nocardiosis occurs worldwide
♦
Infections arise exogenously (unlike Actinomycosis) from inhalation or direct inoculation
♦
Nocardia asteroides complex causes most (about 80–90%) respiratory and disseminated infections
♦
N. brasiliensis and N. otitidis–caviarium cause most cutaneous infections
♦
Predisposing factors include the following:
Leukemia/lymphoma
Immunosuppressive therapy
AIDS
Chronic pulmonary disorders (e.g., pulmonary alveolar proteinosis)
Chronic granulomatous disease of childhood
♦
Pulmonary disease – the predominant clinical manifestations follow:
Fibrinosuppurative pneumonia; usually with minimal fibrosis
Lung abscesses and cavitating lesions (sometimes large)
Empyema
There is a tendency for hematogenous spread originating from the lungs as the primary site to other body sites, particularly the CNS (usually with brain abscess)
♦
Cutaneous or subcutaneous lesions
These result either from traumatic inoculation or from systemic dissemination from the lungs
♦
The treatment of choice for nocardiosis is trimethoprim/sulfamethoxazole
Alternatively, amikacin plus a carbapenem is given in combination intravenously for 3–4 weeks followed by trimethoprim/sulfamethoxazole orally
In contrast, the treatment of choice for actinomycosis is ampicillin, amoxicillin, or penicillin G or penicillin V
Pathology and Stains (Fig. 6.8A–D)
♦
The host response in nocardiosis is usually suppurative necrosis with abscesses, or there may be diffuse fibrinosuppurative pneumonia
♦
In chronic infections, multiloculated abscesses and sinus tracts filled with purulent exudates occur
♦
Fibrinosuppurative pleuritis with empyema is a common complication
♦
In pulmonary and systemic infections, Nocardia species almost never form sulfur granules (unlike Actinomyces israelii), and their thin, branching filaments are not stained with H&E or PAS
♦
In cutaneous mycetomas, Nocardia species form granules
♦
Nocardia filaments stain with Gomori methenamine silver or Grocott stain and with tissue gram stains (particularly Brown–Brenn)
♦
Nocardia forms gram-positive, beaded filamentous rods, usually ≤1 mm in diameter, with more or less right-angle branching
♦
With the Fite stain or the Fite–Faraco stain (modified acid fast) of tissue sections, Nocardia stains partially acid fast or positive
♦
Nocardia also stains partially acid fast using a modified Kinyoun stain for fresh tissue imprints or smears of exudates
Less decolorization occurs with these stains than with the usual Ziehl–Neelsen or auramine–rhodamine acid-fast stains
♦
Actinomyces israelii and the other species that cause Actinomycosis are not acid fast
♦
A negative acid-fast stain does not rule out Nocardia, however
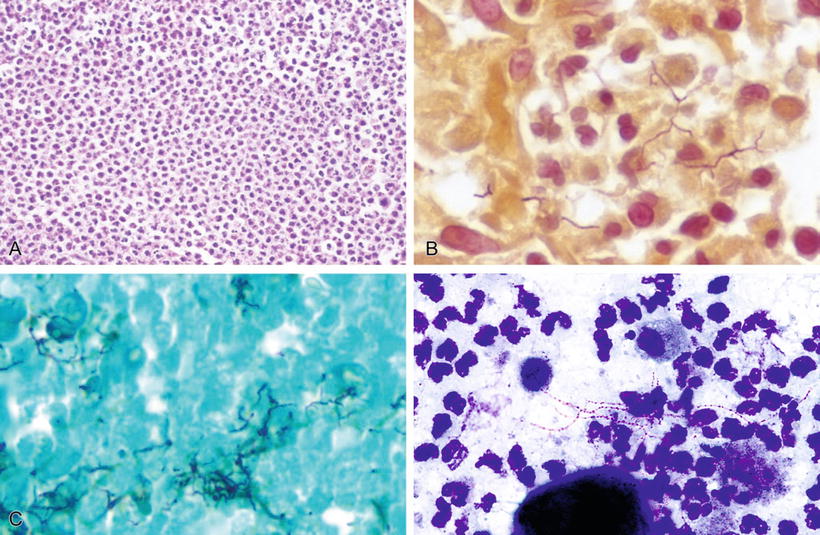
Fig. 6.8.
Pneumonia due to Nocardia species: (A) H&E, (B) Brown-Brenn, (C) GMS, (D) modified acid-fast stain.
Other Diagnostic Techniques
♦
Microbiologic culture is required for definitive identification and susceptibility testing
Rhodococcus equi
♦
R. equi has been previously called “Corynebacterium equi” and “Mycobacterium rhodochrous,” and is also referred to as the “rhodochrous complex”
♦
In culture, this gram-positive organism may first appear rodlike, but then later appear coccoid
♦
Like Nocardia, it is partially acid fast. Unlike Nocardia, it does not form thin, branching, beaded filaments
♦
The colonies of R. equi often have a salmon-pink color on agar media
Clinical
♦
The vast majority of patients (>85%) with R. equi infections are immunocompromised
♦
The most common and clinically important manifestations of these infections include the following:
Cavitary pneumonia
Subcutaneous and soft tissue abscesses
Brain abscesses
Osteomyelitis
Lymphadenitis
Endophth almitis
Macroscopic
♦
Radiographic findings often initially suggest tumor
♦
Lung tissue removed as lobectomy specimens may reveal cavitary lesions surrounded by consolidated lung
♦
Extrapulmonary lesions usually reveal abscesses surrounded by nodular and firm tissue
Microscopic (Fig. 6.9A–E)
♦
Acute suppurative inflammation or granulomatous inflammation may occur
♦
Malakoplakia, a rare chronic granulomatous process seen in R. equi infections of the lung, soft tissue, or elsewhere, may occur with sheets and aggregates of histiocytes containing Michaelis–Gutmann bodies
♦
Granulation tissue may be prominent
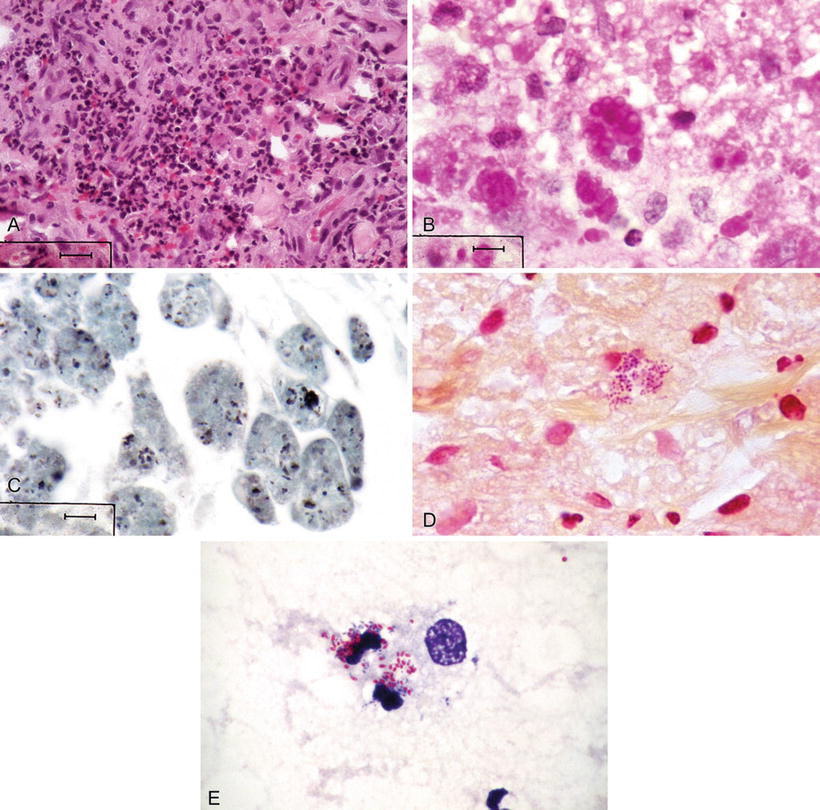
Fig. 6.9.
Rhodococcus equi infection; wall of soft tissue abscess; (A) H&E, (B) PAS-positive inclusions, (C) pleomorphic coccobacilli in macrophages (GMS), (D) gram-positive coccobacilli (Brown–Brenn), (E) partially acid-fast coccobacilli (modified Kinyoun).
Special Stains
♦
PAS stain reveals PAS-positive inclusions in macrophages called Michaelis–Gutmann bodies (which contain lamellated iron and calcium)
♦
Brown–Brenn stain shows a small, gram-positive coccobacillus
♦
Fite or a Fite–Faraco stain of paraffin sections or a modified Kinyoun stain of fresh touch preparations or tissue imprints reveals partially acid-fast coccobacilli. Note: these bacteria will not stain acid fast using Ziehl–Neelsen or auramine–rhodamine stains
♦
Other useful stains include Gomori methenamine silver, Grocott, Giemsa, and Alizarin red
Differential Diagnosis
♦
R. equi is differentiated from Histoplasma capsulatum and other fungi by morphology and culture
♦
It is differentiated from Mycobacterium, species by morphology and microbiologic culture
♦
In pulmonary Whipple disease caused by Tropheryma whippelii, macrophages are more likely to be foamy and not eosinophilic; they are more likely to be found in the pulmonary interstitium and peribronchial smooth muscle
♦
In noninfectious processes (e.g., suppurative bronchiolitis, lipid storage disorders, and exogenous lipid pneumonia), R. equi is differentiated using special stains and microbiologic culture
Gram-Negative Bacilli: Enterobacteriaceae
Escherichia coli
♦
E. coli is a gram -negative, facultatively anaerobic (“aerobic”) rod
♦
It ferments glucose and other carbohydrates and is oxidase negative
♦
It is the most common gram-negative bacillus in the GI tract that grows aerobically
♦
Most infections are endogenous
♦
The organism originates from patient’s own microbiota or “normal flora”
♦
E. coli accounts for about 50% of the bacterial isolates in clinical microbiology laboratories
Clinical
♦
The organism causes extraintestinal infections, both community acquired and nosocomial as listed below
♦
Bacteremia – E. coli is the most common cause of gram-negative rod bloodstream infections
♦
Urinary tract infections (UTIs)
It is the most common cause of UTIs, usually limited to cystitis, but also causes pyelonephritis and prostatitis
♦
Other infections caused by E. coli include:
Neonatal meningitis
Pneumonia
Peritonitis: usually polymicrobial
Wound and soft tissue infections (e.g., cellulitis)
Osteomyelitis and joint infections
♦
Important intestinal illnesses caused by this organism follow:
Traveler’s diarrhea due to enterotoxigenic E. coli (ETEC)
Bloody diarrhea, hemorrhagic colitis, and hemolytic uremic syndrome due to enterohemorrhagic E. coli (e.g., 0157:H7; EHEC) and other Shiga toxin-producing E. coli (STEC) strains
Diarrhea caused by enteropathogenic E. coli (EPEC)
•
A leading cause of infant diarrhea in developing countries
Watery diarrhea in infants in developing countries and in travelers to these countries caused by enteroaggregative E. coli (EAEC)
Enteroinvasive E. coli (EIEC)
•
Food-borne outbreaks in developing countries
Macroscopic
♦
Extraintestinal infections
Apart from clinical settings, there are no distinguishing features of these E. coli associated infections
♦
Intestinal infections – the gross findings depend upon the pathogenic mechanisms involved
Traveler’s diarrhea due to ETEC is associated with abundant watery diarrhea, but without gross pathologic lesions (similar to cholera)
In EHEC diarrhea, the spectrum of findings varies from grossly unremarkable to patchy mucosal ulceration and hemorrhage; the findings are usually more prominent in the right colon
•
Pseudomembranes may be present
Gross findings in EPEC are unremarkable as they are in ETEC diarrhea
In EIEC diarrhea, gross examination may demonstrate patchy mucosal ulceration and hemorrhage
In EAEC, children may have growth retardation due to the chronic diarrhea
Microscopic (Fig. 6.10)
♦
Extraintestinal infections are characterized by acute neutrophilic inflammation
Findings are similar to those in infections involving pyogenic cocci
♦
Intestinal infections
In ETEC diarrhea, the small intestine is microscopically unremarkable as in cholera
•
The colon also is not involved
In EHEC diarrhea, the findings vary from mild nonspecific inflammation to severe hemorrhagic colitis
Findings in EIEC diarrhea can include edema, congestion, focal hemorrhage, and neutrophilic infiltration
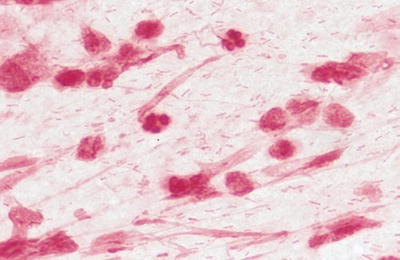
Fig. 6.10.
Escherichia coli in gram-stained smear of sputum.
Special Stains
♦
E. coli usually is in the form of medium-sized gram-negative rods
Brown–Hopps is usually better than a Brown–Brenn tissue gram stain for demonstrating gram-negative rods such as E. coli in tissue sections
♦
PAS is also a useful stain for observing E. coli and other gram-negative bacteria in tissue sections
♦
Diff-Quik and Giemsa stains aid in visualizing E. coli and other gram-negative bacteria in direct smears of body fluids, exudates, and touch preparations of fresh clinical materials
Differential Diagnosis
♦
Extraintestinal E. coli infections are morphologically similar to infections caused by a wide variety of other bacteria
♦
EHEC diarrhea findings are similar to those seen in GI infections caused by Shigella, Salmonella, or Campylobacter species
If pseudomembranes are present, other causes of pseudomembranous colitis (e.g., Clostridium difficile) should be considered
Diagnostic Techniques
♦
Microbiologic culture is used in the clinical microbiology laboratory for identifying E. coli from various types of specimens; culture also is a prerequisite for antimicrobial susceptibility testing
♦
Sorbitol-MacConkey agar for E. coli 0157:H7 is commonly used, but may not detect other STEC
♦
Immunologic testing may be used to detect E. coli Shiga toxin-producing strains
♦
PCR or DNA probes for enterotoxin genes or other virulence genes may be used in public health or reference laboratories
New commercially available PNA-FISH probes and multiplex molecular detection methods may aid in specific and rapid identification of E. coli in positive blood cultures or certain other types of specimens
Shigella Species (Cause Bacillary Dysentery)
♦
Shigella species are gram-negative, facultatively anaerobic, nonmotile rods that genetically are very closely related to E. coli
They are differentiated on the basis of antigenic characteristics and fermentation test results
According to CDC, Shigellosis is the third most common cause of bacterial gastroenteritis in the USA – after Campylobacter and Salmonella
Humans are the only natural hosts
Of all the bacterial diarrheas, shigellosis is the most communicable
It is transmitted by the fecal–oral route
•
Principally by person-to-person spread
•
But also by contaminated food and water
As few as 10–100 viable bacteria cause intestinal illness
♦
In terms of pathogenesis, shigellae pass through the stomach (surviving gastric acid) and the small intestine and cause disease primarily by invading and replicating in mucosal cells of the colon
Most current information on Shigella pathogenesis mechanisms is based on work with S. flexneri
♦
S. flexneri is unable to attach to differentiated mucosal epithelial cells
Instead, it first attaches to and is taken up by microfold cells (M cells)
M cells are specialized epithelial cells that sample particulate and soluble antigens in the gut lumen and present them to underlying lymphoid tissue (e.g., in Peyer patches)
♦
The organism passes through M cells by transcytosis
After transcytosis, it is released into an intraepithelial pocket on the lamina propria side of the M cell where it is engulfed by a macrophage
♦
Shigellae rapidly induce apoptosis in macrophages
Macrophage death is accompanied by release of proinflammatory cytokines IL-1β and IL-18 and attraction of abundant neutrophils to the infected site (characteristic acute inflammation occurs), with epithelial cell lysis, ulceration, and subsequent deeper invasion
♦
Once released from dying macrophages, free Shigellae invade mucosal epithelial cells on the basal lateral side, move into the cytoplasm by directed actin polymerization, and spread to adjacent epithelial cells, thus avoiding extracellular exposure to host immune surveillance
♦
Shigellae (along with E. coli, Salmonella sp., Yersinia sp., and other bacteria) utilize a type III secretion system (plasmid encoded) that mediates secretion of >25 proteins into epithelial cells and macrophages
Once inside target cells, shigellae lyse phagocytic vacuoles, replicate in the cytoplasm, and cause actin to polymerize
Subsequently, they are pushed through the cytoplasm to adjacent mucosal epithelial cells where they continue to replicate
♦
Also, certain strains of S. dysenteriae produce Shiga toxin, a potent exotoxin that inhibits host cell protein synthesis leading to intestinal mucosal damage and necrosis
In addition to its effects in the intestinal tract, Shiga toxin damages glomerular endothelial cells causing renal failure in the hemolytic uremic syndrome
Clinical
♦
Most US cases are caused by S. sonnei
According to CDC there were 208,368 laboratory-confirmed Shigella infections reported to CDC during 1989–2002, of which:
•
S. sonnei (serogroup D) accounted for approximately 72%
•
S. flexneri (serogroup B) accounted for ~18%
•
S. boydii (serogroup C) accounted for ~2%
•
S. dysenteriae (serogroup A) accounted for <1%
S. sonnei usually causes milder disease than does S. flexneri or S. dysenteriae
S. flexneri predominates in the developing world
S. dysenteriae is the most virulent species
♦
Most patients are children (<5 years old) and the range of clinical manifestations includes asymptomatic intestinal carriage or fever, with watery diarrhea or severe dysentery with abdominal cramps, bloody stools, and mucus
♦
Shigella is unusual in infections outside the GI tract though life-threatening bacteremia, hemolytic uremic syndrome, and toxic megacolon manifestations have been documented, and sequelae including reactive arthritis occur
♦
Shigella bacteremia, though the organism is not commonly isolated from blood cultures, is more likely in patients with AIDS or in other immunocompromised patients
♦
Reactive arthritis with urethritis and conjunctivitis (e.g., Reiter syndrome) is a known complication of shigellosis (associated with HLA-B27 haplotype)
Macroscopic
♦
Involvement of the colon is much more pronounced than involvement of the small intestine
♦
The extent of the colonic involvement varies from subtotal to total; it may be continuous, diffuse, or patchy
♦
The most common gross findings are erythema, edema, and superficial ulceration in 40%; pseudomembranes are present in 20%
Microscopic
♦
Edema, congestion, focal hemorrhages, crypt hyperplasia, depletion of goblet cells, neutrophilic infiltration, and ulcers are common
♦
Crypt abscesses and capillary thrombi are also common
Special Stains
♦
Gram-negative rods can be demonstrated in the mucosa and crypts
♦
The bacteria may also be prominent within the lamina propria
♦
Invasion beyond the lamina propria is rare
Differential Diagnosis
♦
The differential includes intestinal illness due to the following
Escherichia coli
Salmonella species
Campylobacter species
Clostridium difficile (pseudomembrane formation)
Diagnostic Techniques
♦
Isolation and identification of the organism from fecal specimens are required for diagnosis
♦
Recovery of isolates depends upon proper use of selective and differential media
Nontyphoidal Salmonella Infections
♦
Salmonellae are gram-negative, motile (with rare exception) rods that ferment glucose but not lactose
Members of the family Enterobacteriaceae, they are closely related phylogenetically to E. coli and Shigella
In the clinical microbiology laboratory, they are identified based on antigenic characteristics (O and H agglutinins) and biochemical test results
Taxonomic classification remains complex, with >2,500 “O” serotypes (referred to as “species”), of a single species now designated as Salmonella enterica
♦
In terms of pathogenesis, these organisms invade the mucosa of the small and large intestine
♦
Salmonellae ingested in food or from fecal–oral transmission survive the gastric acid barrier if the inoculum size is sufficiently high
The infectious dose for most serotypes other than Salmonella typhi and Salmonella paratyphi varies (e.g., 106–108 to 200 bacteria), but lower in patients who are elderly, immunocompromised, those with decreased gastric acidity, and others
♦
Salmonellae that reach the small intestine attach to the mucosa and invade M cells (Peyer patches) and enterocytes
♦
There they replicate within endocytic vacuoles
♦
Attachment, engulfment, and replication are under the control of two large clusters of genes on the bacterial chromosome called pathogenicity islands
Pathogenicity island I encodes a type III secretion system that enables Salmonella to enter
Pathogenicity island II encodes a second type III secretion system that facilitates survival in macrophages
Clinical
♦
The incubation period is usually 6–48 h
♦
Illness occurs primarily in children
♦
There is a range of clinical syndromes
♦
Manifestations include diarrhea, often with fever and abdominal cramps
♦
Typically, the diarrhea lasts 3–7 days
♦
Outbreaks most often are food-borne (e.g., contaminated eggs, ice cream, turkey, chicken, beef)
♦
Waterborne spread also occurs
Pathology (of Food Poisoning)
♦
The manifestations include the following:
Acute ileocolitis, with mucosal erosion
Mixed inflammation within the lamina propria
Presence of neutrophils and red blood cells in patients’ feces
The mucosa usually returns to normal in about 2 weeks
♦
Other Salmonella infections include:
Localized infections (e.g., osteomyelitis associated with sickle cell disease)
Bacteremia (most commonly due to Salmonella typhi, Salmonella paratyphi, Salmonella choleraesuis, and Salmonella enteritidis)
Diagnostic Techniques
♦
The principal means of diagnosis is culture: with isolation and identification of Salmonella serotypes from stool
♦
Selective media are used
Salmonella typhi (Typhoid Fever)
♦
Salmonella typhi causes typhoid fever
♦
A milder form of illness, paratyphoid fever, is produced by Salmonella paratyphi A, Salmonella (paratyphi B) schottmulleri, and Salmonella (paratyphi C) hirschfeldii
Humans are the only reservoirs of Salmonella typhi and Salmonella paratyphi
Chronic carrier states exist for these Salmonellae, which can reside for long periods of time in the gall bladder
Person-to-person spread is common, as the infectious dose for Salmonella typhi is low
Typhoid fever is usually acquired from ingesting food or water contaminated by an infected food handler who is a carrier
♦
In terms of pathogenesis, the organism passes through the stomach (survives the gastric acid)
♦
In the terminal ileum, it is taken up by and invades M cells
♦
Salmonella typhi is then engulfed by macrophages in the lymphoid tissue in Peyer patches
♦
Unlike most other serotypes of Salmonella enterica, Salmonella typhi disseminates via lymphatics and through the bloodstream to the spleen, liver, bone marrow, and other sites
Clinical
♦
This is a severe systemic infection referred to as “enteric fever” – characterized by fever and abdominal symptoms
♦
The incubation period typically is 7–14 days
♦
Symptoms include headache, malaise, abdominal pain and tenderness, constipation, and myalgias, with mental status changes
♦
Fever is a classic sign of typhoid fever, but does not always develop
♦
“Rose spots” (maculopapular rash on the trunk) develop in 30–50% of patients
♦
Cervical lymphadenopathy may be prominent
♦
About half of patients develop hepatosplenomegaly
Macroscopic
♦
Enlarged lymphatic submucosal nodules in the small intestine and colon may be a prominent finding, especially in Peyer patches of the terminal ileum (where ulcers with the long axes in the direction of bowel flow may be seen)
Microscopic
♦
Typhoid nodules and focal collections of macrophages (macrophages with erythrophagocytosis, debris and bacteria) may be seen (e.g., in the liver and/or spleen)
♦
Salmonellae may localize in the conjunctivae, meninges, joints, kidneys, and gallbladder (3% carriers)
♦
Pneumonia is a frequent complication (superinfection)
Diagnostic Techniques
♦
Microbiologic cultures of blood, stool, and urine are indicated
In the first week of typhoid fever, 80% of blood cultures are positive, with decreasing rates of blood culture positivity in subsequent weeks
The causative organism is more likely to be isolated from stool or urine in the second week of illness rather than during the first week
Appropriate selective isolation media are required for culturing stool and urine
♦
Other specimens cultured may include rose spots and/or bone marrow
♦
In addition to testing an isolate suspected of being Salmonella serotype typhi for agglutination using somatic O group D antiserum (which contains antibodies to antigens 9 and 12), testing for the Vi antigen, a heat-labile polysaccharide, is useful
Yersinia enterocolitica
♦
Y. enterocolitica is a member of the family Enterobacteriaceae and a facultatively anaerobic, nonlactose-fermenting gram-negative rod or coccoid rod (0.5–0.8 μm × 1–3 μm)
Pigs are a major reservoir
Y. enterocolitica also has been isolated from dogs, cats, and various other animals
It causes gastrointestinal illness in humans much more frequently than does Y. pseudotuberculosis
The pathogenesis of enteric infections with this organism bears some similarity to that of salmonellosis
♦
The pathogenicity of Y. enterocolitica is related to virulence factors encoded by a virulence plasmid (pYV) and by the bacterial chromosome
♦
In the terminal ileum or colon, the organism uses adhesins to adhere to M cells
♦
In a manner similar to Salmonella, it uses a type III secretion system (containing pYV encoded proteins)
♦
Following adherence, Y. enterocolitica penetrates through enterocytes into the lamina propria
♦
Inside a cell, it is enclosed within membranous vesicles
Clinical
♦
In the USA, Y. enterocolitica is an infrequent cause of diarrhea and right lower quadrant abdominal pain (which may mimic acute appendicitis clinically)
♦
It is more common in Northern Europe, South America, Africa, and Asia
♦
The incubation period is usually 4–6 days
♦
Food-borne outbreaks have been associated with various foods (e.g., associated with chocolate milk in New York, pasteurized milk in Tennessee, raw pork intestines [chitterlings] in Atlanta and Baltimore)
♦
Cases of Y. enterocolitica sepsis related to the transfusion of red blood cells (RBCs) have been reported. Y. enterocolita grows well at the refrigerator temperatures where RBCs are stored
Microscopic (Fig. 6.11)
♦
Yersinia enterocolitis may involve the terminal ileum and colon (i.e., as an acute self-limited enterocolitis) or as an acute terminal ileitis (i.e., with the terminal ileum as the main focus)
♦
Intestinal lesions may be similar to those seen in typhoid fever (with ulceration involving Peyer patches), but intestinal perforation is rare
♦
Enlarged mesenteric lymph nodes with “stellate abscesses” are characteristic; the histologic findings are similar to those in lymphogranuloma venereum or the lymph nodes in cat-scratch disease
♦
The bacterium has been implicated as an uncommon cause of acute appendicitis
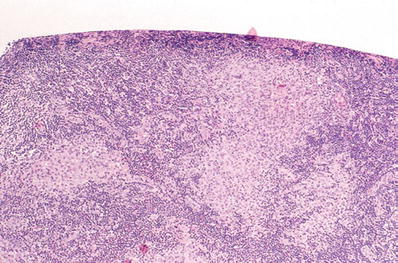
Fig. 6.11.
Yersinia enterocolitica; mesenteric lymph node (H&E).
Special Stains
♦
Gram stain (using Brown–Hopps procedure) reveals gram-negative coccobacilli
♦
Silver stains (e.g., Warthin–Starry) may be useful for demonstrating organisms
♦
Immunohistochemical stains have sometimes been used
Differential Diagnosis
♦
The differential based on morphologic findings alone is broad and includes the following:
Typhoid
Tuberculosis
Pseudotuberculosis
Brucellosis
Intestinal tularemia
Lymphogranuloma venereum
Crohn disease
Microbiologic Culture
♦
A cold enrichment technique (enrichment buffer at 4 °C) along with selective media can be used for primary isolation
♦
Yersiniae grow well at refrigerator temperatures whereas other bacteria in feces usually grow less well in the cold; in addition, yersiniae are motile at 25 °C but not at 35 °C
Yersinia pestis
♦
Y. pestis is an extremely virulent pathogen that causes plague
♦
It has two plasmids carrying virulence genes that encode for:
An antiphagocytic capsule
Plasminogen activator protease
•
Degrades complement components C3b (prevents opsonization) and C5a (prevents phagocytic migration)
•
Plasminogen activator also degrades fibrin clots (enhancing spread of Y. pestis)
♦
Virulence of Y. pestis is enhanced by iron
A pathogenicity island encodes a siderophore-independent iron uptake system (E. coli, Salmonella, Klebsiella, and Enterobacter have similar systems)
Thus, patients with hemochromatosis or hemolytic anemia are at increased risk for septicemia and death if infected by Yersinia
Clinical
♦
Natural reservoirs includes rats and ground squirrels
♦
Spread of organism: flea bites, direct contact with infected materials or by inhalation of aerosols from individuals with pneumonia
♦
Human plague (mortality is high)
Bubonic
•
Patients have high fever
•
Painful, swollen lymph node with hemorrhagic lymphadenitis – (usually groin or axilla)
Primary and secondary pneumonic
Primary and secondary septicemic
Special Stains
♦
Gram-negative bacillus or coccobacillus with characteristic bipolar (“safety pin”) staining can be helpful, but not diagnostic
Diagnostic Techniques
♦
Specimens to collect include aspirates (e.g., of a bubo), tissue (e.g., lymph nodes), sputum, bronchoalveolar lavage, blood culture, or CSF
The organisms grow on ordinary microbiologic culture media
♦
Direct microscopic examination of gram-stained smears or sections of clinical materials typically reveals small, ovoid gram-negative rods (0.5 μm × 1–2 μm) as single cells, in pairs, or in short chains with bipolar-staining resembling “closed safety pins”
Giemsa stain may aid in revealing this morphology, particularly if gram stains are difficult to interpret
♦
Y. pestis is one of the Category A agents of bioterrorism
These are agents that pose the greatest threat relative to their infectious nature, ease of spread, or high mortality (e.g., Bacillus anthracis, Clostridium botulinum [botulinum toxins], Francisella tularensis, Yersinia pestis, variola major [smallpox], filoviruses [Marburg virus, Ebola virus], and arenaviruses [Lassa virus, Machupo virus])
♦
In a healthcare setting, laboratory procedures to be performed with Y. pestis must be done in a facility with a biosafety level of at least 2
Activities that are at high risk for creating aerosols with this organism should be done in a biosafety level 3 laboratory
♦
If plague is suspected in a clinical laboratory or sentinel laboratory, local and state public health authorities must be notified immediately
♦
The Laboratory Response Network toll-free HelpDesk ((866) 576–5227) is available for subject matter experts if discussions on individual organisms are needed
♦
Another excellent source of laboratory information relative to these types of agents is the American Society for Microbiology website (http://www.asm.org/Policy/index.asp?bid=520)
Klebsiella pneumoniae
♦
Also a member of the family Enterobacteriaceae, K. pneumoniae is a nonmotile, gram-negative rod that ferments lactose
♦
Most strains encountered in the clinical laboratory produce a prominent polysaccharide capsule that is responsible for the mucoid appearance of the colonies on agar media
♦
The capsule also is an important virulence factor of this bacterium; it contributes to the mucoid appearance of “currant jelly” sputum
♦
K. pneumoniae inhabits the gastrointestinal tract; it also colonizes the upper respiratory tracts of approximately 5–10% of healthy individuals
Clinical
♦
The lobar pneumonia historically known as “Friedländer pneumonia” is caused by K. pneumoniae
♦
K. pneumoniae, along with K. pneumoniae subsp. oxytoca, commonly cause community-acquired and nosocomial infections
♦
Predisposing factors include diabetes mellitus, alcoholism, chronic obstructive pulmonary disease, and other underlying illnesses
♦
Common infections include:
Pneumonia
Urinary tract infections
Bacteremia
Surgical wound infections and soft tissue infections
Microscopic
♦
Pyogenic pneumonia is characteristic; it may be patchy, lobular, or lobar
♦
An entire lobe may be consolidated as in lobar pneumonia due to S. pneumoniae
♦
Although pneumonia caused by K. pneumoniae may be similar to that caused by S. pneumoniae, the pneumonia that is due to K. pneumoniae tends to be more destructive (with abscess formation and empyema)
♦
Special stains reveal rods varying from short and ovoid to relatively large rods, often surrounded by halolike capsules (Fig. 6.12)
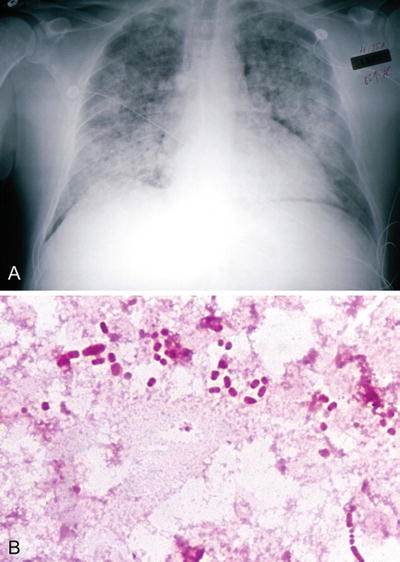

Fig. 6.12.
Klebsiella pneumoniae: (A) chest X-ray, (B) positive blood culture (gram).
♦
These gram-negative bacilli are demonstrated best in tissue sections using Brown–Hopps gram stain
Diagnostic Techniques
♦
Pathologic features of K. pneumoniae infections are not specific
♦
Microbiologic culture is required for definitive diagnosis
Infections Caused by Other Klebsiellae
Rhinoscleroma
♦
Rhinoscleroma is an unusual granulomatous infection of the upper respiratory tract (e.g., involving the nose, nasopharynx, pharynx, or epiglottis) of patients usually from outside the USA (e.g., Central and South America, parts of Africa, the Middle East, India, and the Philippines)
♦
It may be seen in immigrants to the USA, especially those who are immunosuppressed (e.g., due to AIDS)
♦
Infections can be severe with airway compromise or obstruction, the infection can spread contiguously, and it can disseminate via the bloodstream
♦
The causative agent, K. pneumoniae subsp. rhinoscleromatis, is a biochemically inactive strain of K. pneumoniae
♦
It is not a normal member of the upper respiratory tract microbiota
Microscopic
♦
H&E-stained sections reveal sheets of prominent foamy macrophages interspersed with lymphocytes and plasma cells
The overlying mucosa may show pseudoepitheliomatous hyperplasia, or it may be thin or otherwise unremarkable
♦
Although this is a granulomatous infection with sheets of or focal collections of macrophages, well-formed granulomas like those seen in tuberculosis (e.g., tuberculoid granulomas) are not seen
♦
“Mikulicz cells”
Recognition of these foamy macrophages with eccentric nuclei laden with characteristic intracytoplasmic ovoid-to-rod-shaped bacteria (called Mikulicz cells) is an important clue to the diagnosis
The number of Mikulicz cells in lesions varies considerably from few to many
PAS stain is more useful for demonstrating these intracellular bacteria than Brown–Hopps or Brown–Brenn gram stains
GMS is not useful for demonstrating these bacteria, and the organisms are not acid fast
Ozena
♦
Ozena is a chronic atrophic rhinitis caused by K. pneumoniae subsp. ozaenae
♦
It is not common in the USA
♦
The organism is a biochemically inert strain of K. pneumoniae
Granuloma inguinale
♦
This is a sexually transmitted granulomatous infection of the genitalia and inguinal area that can be confused with the chancre of primary syphilis
♦
The infection is uncommon in the USA, but relatively common in areas of the Caribbean, South America, India, Vietnam, parts of South Africa, Australia, and Papua New Guinea
♦
The causative agent, now called Klebsiella granulomatis, was previously named Calymmatobacterium granulomatis; before that it was called Donovania granulomatis
♦
In relation to its former genus epithet, the old name “donovanosis” has persisted for many years, though granuloma inguinale is the preferred name for the infection
♦
K. granulomatis cannot be cultured in vitro in microbiologic culture media
Other Gram-Negative Bacilli
Campylobacter jejuni
♦
This is a small (0.2–0.5 μm × 0.5–5 μm), motile (by means of polar flagella), comma-shaped gram-negative bacterium that is microaerophilic
♦
It grows optimally in an atmosphere of 5–10% oxygen plus 10% carbon dioxide and the rest nitrogen
♦
An important cause of zoonotic infections, Campylobacter species commonly inhabit the intestines of poultry, domestic animals (e.g., C. upsaliensis can be isolated from dog feces including pets with diarrhea), and wild animals
♦
Both food-borne and waterborne outbreaks of Campylobacter intestinal illness are common
♦
Fecal–oral spread also occurs
♦
Although putative virulence factors of this invasive organism have been described, including surface adhesins, enterotoxins, and cytotoxic enzymes, information is lacking regarding their role in disease
Clinical
♦
C. jejuni currently is the most common bacterial cause of enteritis in the USA
♦
The spectrum of illness ranges from mild diarrhea to moderately severe enteritis
At the extreme, there may be fatal colitis with bloody and/or purulent stools
♦
Abdominal pain frequently is the predominant symptom
♦
Clinically significant bacteremia may also be caused by Campylobacter species
C. fetus is the Campylobacter species most commonly implicated in bacteremia
Bacteremia occurs in ≤1% of patients with C. jejuni infection
♦
Guillain–Barré syndrome (GBS) is an uncommon complication of Campylobacter jejuni or C. upsaliensis infections
GBS is thought to be an autoimmune disease due to antigenic cross-reactivity between surface lipopolysaccharides of Campylobacter spp and peripheral nerve gangliosides
However, GBS has also been reported following certain viral infections (e.g., CMV, certain enteroviruses, influenza virus) and in about 1 in 100,000 recipients of the swine flu vaccine during the 1976 vaccination campaign
Macroscopic
♦
The colon may show erythema and friability
♦
The infection may resemble idiopathic ulcerative colitis or Crohn disease
Microscopic
♦
Findings may include cryptitis (neutrophil infiltration), crypt abscesses, preservation of crypt architecture, capillary congestion, and edema in the lamina propria
♦
Other nonspecific findings may range from “unremarkable” or mild inflammation to terminal ileitis or pseudomembranous colitis
Special Stains
♦
Direct microscopic examination of gram-stained material from positive blood cultures or other microbiologic cultures reveals curved gram-negative rods, “s,” or “gull-wing”-shaped bacteria
♦
Direct microscopic examination of feces for Campylobacter lacks sensitivity; specificity depends upon the experience and skill of the microscopist
♦
Morphologic forms of Campylobacter sp. are seldom documented in histologic sections
Differential Diagnosis
♦
Campylobacter gastroenteritis can be confused with idiopathic inflammatory bowel disease
♦
Other infectious colitides including Salmonellosis or Shigellosis
Diagnostic Techniques
♦
Microbiologic culture requires the use of special media and the incubation of the inoculated media at elevated temperature (i.e., 42 °C), under microaerophilic atmospheric conditions
Vibrio vulnificus
♦
V. vulnificus is a motile (by means of polar flagella), gram-negative, rod-shaped, and curved, oxidase-positive, and lactose-positive, free-living marine bacterium
♦
It occurs in the coastal waters of the Gulf of Mexico, the Atlantic and Pacific oceans, and also the Great Salt Lake in Utah as well as in other lakes (e.g., in New Mexico and Oklahoma)
♦
Responsible for 90% of the deaths due to Vibrio infections in the USA, V. vulnificus is the most virulent noncholera Vibrio species
♦
A polysaccharide capsule and certain enzymes are its principal virulence factors
Clinical
♦
Primary septicemia
Septicemia due to V. vulnificus has been especially severe in patients with known liver disease (75% in one study), other patients with underlying hematopoietic illness, and patients with renal failure or who are immunocompromised and eat raw oysters or other shellfish containing the organism
Secondary skin lesions occur in 90% (usually ecchymoses, bullae, and necrotizing cutaneous and soft tissue lesions)
The mortality associated with V. vulnificus septicemia is high (~50%)
♦
Wound infections
Seawater exposure to open wounds or injury (e.g., while fishing, swimming, shucking oysters, and other activities) is a major risk factor
Findings in these infections include erythema, swelling, intense pain, cellulitis, fasciitis, and/or myositis or gas gangrene, particularly involving the lower or upper extremities
Bacteremia occurs in ~30% of patients who have a V. vulnificus primary wound infection
•
The mortality is lower for these patients than that of patients with primary septicemia
Mortality for those with V. vulnificus primary wound infections and underlying liver disease (e.g., cirrhosis) is about 25%
•
The prognosis is better if the patients do not have liver disease
♦
Gastroenteritis (by itself)
Vomiting, diarrhea, and abdominal pain occur in approximately 10% of patients
The prognosis is generally excellent (i.e., no associated mortality)
Microscopic (Fig. 6.13A, B)
♦
Skin lesions from patients with primary septicemia and skin lesions resulting from seawater – related wound infections are similar; the findings include the following:
Intense neutrophilic infiltration of subcutaneous tissue
Abundant bacteria within the inflammatory cell infiltrate
Acute vasculitis and thrombosis with a haze of many bacteria in vessel walls
Acute necrotizing inflammation involving the dermis
♦
In addition, there may be areas of intense fasciitis, myositis, necrotizing vasculitis, and myonecrosis
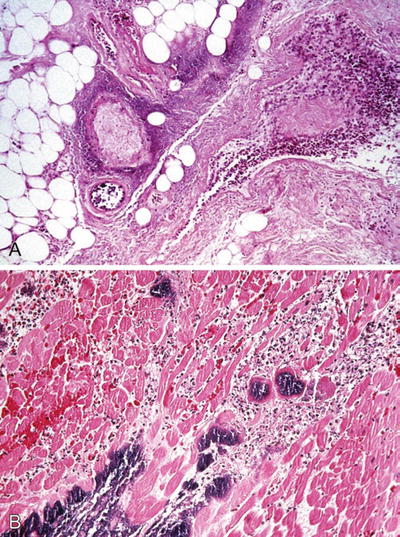
Fig. 6.13.
Vibrio vulnificus wound infection with (A) vascular necrosis and cellulitis (H&E), (B) skeletal muscle necrosis, and neutrophilic infiltrate (H&E).
Special Stains
♦
The organisms are gram-negative comma-shaped bacilli
♦
Brown–Hopps tissue gram stain can be particularly useful and is preferable to the Brown–Brenn tissue gram stain for these bacteria
Differential Diagnosis
♦
The necrotizing skin and soft tissue lesions are similar to those produced by Clostridium species, Aeromonas species, and Streptococcus species, as well as polymicrobial infections involving Bacteroides species, the anaerobic and microaerophilic cocci, as well as other anaerobic and nonanaerobic microorganisms
♦
The “cloud-like” appearance of bacteria in vessel walls and similar vascular lesions can be seen in necrotizing infections caused by Pseudomonas aeruginosa
Diagnostic Techniques
♦
Microbiology laboratories should be notified if V. vulnificus or another Vibrio is suspected
♦
Thiosulfate citrate bile salts sucrose (TCBS) agar is the primary isolation medium of choice, but is not available in all laboratories, particularly those that are geographically far from coastal areas
♦
Even though V. vulnificus is a halophilic, oxidase-positive, and lactose-positive Vibrio, it grows on many if not most nonselective blood agar media and on routinely used enteric selective plating media (e.g., MacConkey, xylose lysine deoxycholate, and Hektoen-enteric agar), as well as in most blood culture media. It can be confused with lactose-positive Enterobacteriaceae
Pseudomonas and Related Genera
General Characteristics
♦
These bacteria are ubiquitous in the environment (e.g., in/on flowers, sinks, toilets, and respiratory therapy equipment in hospitals) and in upper respiratory and intestinal microbiota of hospitalized patients
It can contaminate disinfectant solutions, distilled water, and stain solutions
♦
Most occur as small, thin, gram-negative rods; they are motile by means of polar flagella
♦
These are nonfermenters; they utilize glucose oxidatively
♦
Most of these bacteria produce cytochrome oxidase (Enterobacteriaceae are oxidase negative)
♦
The many virulence factors produced by Pseudomonas aeruginosa are summarized as follows:
Adhesins (e.g., flagella, pili, lipopolysaccharide, alginate capsules)
Various toxins and enzymes (e.g., exotoxin A, pyocyanin, pyoverdin, LasA [a serine protease] and LasB [a zinc metalloprotease], alkaline protease, phospholipase C, and exoenzymes S and T)
Antibiotic resistance
•
P. aeruginosa is inherently resistant to many antibiotics
•
Porin protein mutations cause resistance to many antibiotics
•
This organism also produces a number of β-lactamases that inactivate penicillins, cephalosporins, and carbapenems
In addition, type III secretion system is used by P. aeruginosa to deliver its toxins into host cells
Pseudomonas Aeruginosa
♦
Bacteremia/septicemia
P. aeruginosa bacteremia is most common in patients with neutropenia, diabetes mellitus, severe burns, and hematologic neoplasms and in patients who have undergone transplant procedures
The most common underlying infection sites leading to bacteremia are pulmonary, urinary tract, and skin
Ecthyma gangrenosum is a well-demarcated, hemorrhagic, and necrotic skin lesion with vascular necrosis and large numbers of bacteria that occurs in bacteremic patients with neutropenia
♦
Skin infections
Burns – colonization of burns by P. aeruginosa may be followed by localized necrotizing vasculitis and bacteremia
Folliculitis – exposure to P. aeruginosa in hot tubs, whirl pools, and swimming pools can predispose individuals to folliculitis
♦
Chronic, suppurative, external otitis (swimmer’s ear) may also be due to P. aeruginosa
♦
Pulmonary infections
P. aeruginosa is common in cystic fibrosis patients
The findings vary from asymptomatic colonization to life-threatening, necrotizing bronchopneumonia
Microscopic features include the following: necrotizing inflammation with large numbers of neutrophils and masses of bacteria in and around walls of thrombosed vessels
Chronic infection may lead to bronchiectasis and pulmonary fibrosis
♦
Urinary tract infections due to this organism occur mostly in catheterized patients
♦
Other infections caused by P. aeruginosa
Keratitis in wearers of contact lenses or after corneal trauma
Endocarditis or osteomyelitis
•
These infections due to this organism are seen primarily in intravenous drug abusers
Burkholderia (Formerly Pseudomonas) cepacia
♦
Several characteristics of this organism are shared with P. aeruginosa
B. cepacia causes bacteremia/septicemia (often catheter related and polymicrobial)
It causes pulmonary infections
•
Frequently in patients with cystic fibrosis or chronic granulomatous disease
•
The findings range from simple colonization to necrotizing pneumonia
B. cepacia also causes skin and soft tissue infections associated with burns or surgical wounds
Burkholderia mallei
♦
This organism causes glanders, a highly communicable disease of livestock (especially in horses, mules, and donkeys)
♦
It has been eradicated from many countries around the world, except in the Middle East, Africa, Asia, and South America where enzootic foci persist
♦
B. mallei can also be transmitted to humans
♦
Although there has only been one human case in the USA during the past half century (involving a biodefense laboratory worker), B. mallei has been identified as a potential agent of bioterrorism
Burkholderia pseudomallei
♦
This is the cause of human melioidosis
♦
The organism is acquired in humans primarily in wounds or following nontraumatic exposure to water or soil
♦
It is very rare in the Western hemisphere
♦
Endemic areas include Vietnam, Indonesia, Thailand, Malaysia, Singapore, China (especially Hong Kong), Taiwan, Cambodia, Laos, the Philippines, India, Pakistan, Papua New Guinea, Fiji, tropical Australia, and parts of Africa
♦
Person-to-person spread is unusual
Laboratory infection has been documented but it is rare
♦
Clinical findings range from asymptomatic infection to localized cutaneous ulcers or abscesses, chronic pneumonia with features similar to tuberculosis, or septicemia with metastatic abscesses involving multiple organs. Symptoms may appear years (sometimes decades) following initial exposure
♦
Gram stain of the organism from culture may demonstrate similar “safety pin” morphology as Y. pestis
♦
Histopathologic findings include:
Abscesses
Necrotizing and nonnecrotizing granulomas
Stenotrophomonas maltophilia
♦
This organism is rarely pathogenic for healthy individuals, but is a relatively common cause of opportunistic infections including wound infections in debilitated or immunocompromised hosts
♦
It is a significant cause of hospital-acquired infections, associated with high morbidity and mortality
♦
It is an important cause of pneumonia, particularly in cystic fibrosis patients, and it is a potential problem for patients on ventilators in intensive care units
♦
It also is an important cause of life-threatening bacteremia, particularly in patients with central venous catheters
♦
Other less frequent infections due to this organism include endocarditis, sinusitis, cellulitis, liver abscess, and meningitis
♦
Like Pseudomonas aeruginosa, S. maltophilia is resistant to many antibiotics
Acinetobacter baumannii
♦
The organism occurs as gram-negative coccobacilli that may be in the form of cocci that are easily confused with Neisseria species
♦
Acinetobacter species cause septicemia, wound infections, pulmonary, and urinary tract infections
♦
They are often resistant to multiple antibiotics and treatment can be problematic
Legionella pneumophila
♦
L. pneumophila is a small (0.3–1 μm × 2 μm), pleomorphic, aerobic gram-negative rod that is nutritionally fastidious. It is ubiquitous in aquatic habitats (e.g., lakes, rivers, streams)
♦
Its presence in cooling towers and condensers of air conditioning systems and in potable water systems (e.g., pipes, water heaters) has been linked to both outbreaks and sporadic cases of legionellosis, some of which have been linked to hospitals
♦
Aerosols (e.g., from showers) constitute the usual route of transmission
♦
As facultative intracellular parasites, Legionella multiply within free-living amebae in aquatic habitats
They multiply within monocytes and macrophages in infected humans
♦
Phagolysosome fusion is inhibited within infected macrophages; inside macrophages Legionella survive exposure to toxic peroxides, superoxide dismutase, and hydroxyl radicals
♦
Legionella produce proteolytic enzymes, lipase, and phosphatase
The infected macrophages release cytokines which stimulate a vigorous inflammatory response
Clinical
♦
Legionnaires disease (legionellosis) is a clinically severe pneumonia with prominent morbidity
Mortality in previously healthy people is 15–20%
Mortality due to legionellosis in immunocompromised patients is 75%
♦
Extrapulmonary involvement is possible
♦
Patients may have high temperature ≥40 °C, diarrhea, and hyponatremia
♦
Pontiac fever is a less severe, self-limited, flu-like illness
Macroscopic
♦
Grossly, pneumonia due to Legionella may be in the form of focal, lobular, or lobar consolidation involving one or multiple lobes. Abscesses or cavitary lesions may be present
Microscopic
♦
Microscopic findings vary from predominantly acute inflammation (with infiltrates containing abundant neutrophils), infiltrates composed mostly of macrophages, or mixed inflammatory cell infiltrates containing a mixture of neutrophils, macrophages, and some lymphocytes
♦
Necrosis may be severe in some cases
♦
Vasculitis with or without thrombi may also be seen
Microscopic Examination Using Special Stains
♦
Using Brown–Hopps tissue gram stains, these gram-negative rods are usually coccobacillary
The organisms may be missed or difficult to see
♦
In tissue, Legionella will stain with nonspecific silver stains (e.g., Dieterle, Steiner, or Warthin–Starry)
♦
Giemsa and gram stains are useful for direct examination of fresh clinical materials
♦
In fresh clinical materials, Legionella micdadei can stain partially acid fast using a modified Kinyoun acid-fast stain
This stain uses a weaker acid than does the Ziehl–Neelsen acid-fast stain
Isolates of L. micdadei may retain or lose their partial acid fastness in vitro in culture media
Diagnostic Techniques
♦
Direct fluorescent antibody (DFA) stains used for direct examinations of clinical specimens have a relatively poor sensitivity (e.g., 25–75%)
♦
Urinary antigen assays are used to detect the lipopolysaccharide antigen of L. pneumophila serogroup 1 (for which the sensitivity is about 60–90%)
♦
Culture on buffered charcoal yeast extract is recommended
Note that for primary isolation, supplementation of the agar medium with l-cysteine is required, and growth is enhanced by supplementation with iron and α-ketoglutarate
In addition, activated charcoal is added to protect the organism from toxic peroxides and lipids, and an organic buffer (N-[2-acetamido]-2-aminoethanesulfonic acid, ACES) is added to protect against toxic NaCl concentration and to maintain the necessary pH
♦
For clinical isolates, gram stain features, colony characteristics, DFA stains of growth from colonies, fluorescence under UV light, and certain biochemical tests aid in presumptive diagnosis of L. pneumophila
For definitive diagnosis, gas–liquid chromatography of cellular fatty acids and nucleic acid sequencing are required
Haemophilus influenzae
♦
These are small, nonmotile, pleomorphic gram-negative rods
♦
Nontypeable strains colonize the upper respiratory tracts of 30–80% of healthy individuals
♦
Encapsulated H. influenzae type B colonization has decreased substantially (now in <1%) since the vaccine was introduced
Clinical
♦
Illnesses caused by nontypeable H. influenzae include the following:
Otitis media (the organism causes about 25% of the 25 million episodes per year in the USA)
Exacerbations of COPD – it is the most common bacterial cause of exacerbations of chronic obstructive pulmonary disease
Community acquired pneumonia – it is relatively common in adults
Pneumonia in children – H. influenzae is a major cause of childhood pneumonia in developing countries
Acute maxillary sinusitis (sinus aspirates are required for diagnosis)
Neonatal sepsis; this has had an increasing frequency since the 1980s; it is associated with significant morbidity and mortality
Purulent conjunctivitis – it occurs sporadically and in outbreaks
♦
Clinical illnesses caused by Haemophilus influenzae type B include the following:
Pyogenic meningitis
•
This is the most serious form of illness caused by H. influenzae
•
It is now far less frequent than before the vaccine was introduced and occurs most often in incompletely immunized persons
•
No clinical feature distinguishes it from purulent meningitis caused by other microorganisms
•
At autopsy, the exudate is usually basal; in pneumococcal meningitis the exudate is usually more prominent over the cerebral convexities near the sagittal sinus
Acute epiglottitis
Pneumonia and empyema
Cellulitis
Bacteremia
Septic arthritis
Diagnostic Techniques
♦
Microbiologic studies (i.e., direct microscopic examination and culture) are required
Bordetella pertussis
♦
B. pertussis, the etiologic agent of whooping cough, is a very small (0.2–0.5 μm × 0.5–1 μm) gram-negative coccoid rod
♦
It is an obligate aerobe that is unable to ferment carbohydrates
It oxidizes amino acids to obtain energy
♦
The organism produces several virulence factors including adhesins that aid in binding of the organism to cilia or host cells (e.g., filamentous hemagglutinin, pertactin, and fimbriae) and toxins (e.g., pertussis toxin, adenylate cyclase/hemolysin, and dermonecrotic toxin)
♦
The vaccines currently used in the USA contain inactivated pertussis toxin, filamentous hemagglutinin, and pertactin
Clinical
♦
Whooping cough (or pertussis) is acquired by inhalation of infectious aerosols followed by attachment of the bacteria to and replication on ciliated bronchial epithelial cells. B. pertussis also invades macrophages. Humans are the only reservoir
♦
Infants 1–2 months of age are at greatest risk
In the developing world where vaccination is lacking, mortality due to whooping cough ranges in the hundreds of thousands
♦
In recent years in the USA, whooping cough has become more common in older children and adults
This trend has occurred in an era in which vaccination rates have been high
The most likely reason for this trend is waning immunity
♦
After a 7–10-day incubation period, pertussis occurs as a three-stage illness summarized as follows:
Stage 1 – catarrhal
•
1–2 weeks
•
The patient has nonspecific upper respiratory symptoms resembling a cold
•
Pertussis is highly communicable at this stage
Stage 2 – paroxysmal
•
2–4 weeks or more
•
There are increased severity and frequency of cough with the classic inspiratory “whoop.”
•
A marked lymphocytosis is present in stage 2
Stage – convalescent
•
3–4 weeks
•
There is decreased coughing
•
Complete recovery occurs in weeks to months
Microscopic
♦
The findings are characterized by laryngotracheobronchitis with abundant mucosal exudate containing inflammatory cells and sloughed, degenerated, mucosal epithelial cells
♦
Segments of hyperemic, denuded bronchial wall may be present
♦
An inflammatory infiltrate involving alveoli would most likely be secondary to superinfection
Special Stains
♦
Brown–Hopps may reveal very small gram-negative coccobacilli
Diagnostic Techniques
♦
A nasopharyngeal aspirate is the specimen of choice
If swabs are to be used, they should be calcium alginate or dacron
•
Cotton swabs contain fatty acids, which are toxic for the organisms
To collect sufficient numbers of cells, posterior nasopharyngeal samples (not oropharyngeal) are necessary
♦
A direct fluorescent antibody (DFA) test, done in conjunction with culture, is currently used in some diagnostic laboratories
The DFA has poor sensitivity and specificity and should not be used by itself
♦
Bordet–Gengou agar, traditionally used for primary isolation of B. pertussis, has been replaced by supplemented Regan–Lowe charcoal medium
The inoculated media require lengthy incubation (7–12 days) at 35 °C
♦
More sensitive nucleic acid amplification tests (e.g., PCR) are replacing the DFA test and culture in diagnostic laboratories that have the necessary resources
Because no FDA-approved molecular test had become commercially available until recently for this organism, laboratories sometimes developed their own procedures targeting various genes
Brucella Species and Brucellosis
♦
Brucella species are small, gram-negative, nonmotile, coccoid rods
♦
They are obligate aerobes that grow very slowly on complex media; do not ferment carbohydrates
♦
As facultative intracellular parasites, Brucella species inhibit phagolysosomal fusion and resist intraphagocytic killing by macrophages
♦
Brucellosis is an extremely important zoonosis, infecting both humans and a variety of animals around the world
♦
Endemic infections due to these bacteria are prevalent in the Mediterranean basin, Middle East, India, Latin America, the Caribbean, Africa, and Asia
♦
Humans can acquire the infection in several ways
Through skin contact (e.g., through a wound or abrasion)
Through a mucosal surface
Ingestion of contaminated food
Through infectious aerosols
Clinical
♦
Different Brucella species are acquired by (examples follow)
Consumption of unpasteurized goat cheese or goat milk (B. melitensis)
Drinking unpasteurized cow milk (B. abortus)
Occupational exposures to infected cattle by butchers, meat cutters, veterinarians, laboratory workers, and others (B. abortus)
Exposure to infected swine (B. suis)
Owners of pet dogs (B. canis)
♦
Laboratory workers are at risk of acquiring the infection through:
Improper handling of specimens (e.g., due to exposure of unprotected skin or mucous membranes)
“Sniffing” of culture plates
Mouth pipetting
Aerosols (e.g., exposures to the eyes, nose, or mouth)
♦
The incubation period varies (1–8 weeks)
♦
Brucellosis is characterized by:
Nonspecific fever, chills, sweats, headache, and malaise, with or without hepatosplenomegaly, lymphadenopathy, anorexia, nausea, vomiting, abdominal pain, diarrhea, and constipation
Sudden death (rare) may occur within days or weeks of delirium and coma
Recurrent “undulant” fever with intermittent or periodic nocturnal fevers is the most common form; patients are likely to have skeletal involvement
There also may be a chronic intermittent form with vague symptoms, mild fever, and profound weakness
Complications include meningitis, endocarditis (the most common fatal complication), pericarditis, hepatic and splenic lesions, osteomyelitis, arthritis, and sacroiliitis
Microscopic
♦
The organisms are sequestered in the reticuloendothelial system
Thus, the lymph nodes, spleen, liver, and bone marrow are commonly involved
Pathologic findings vary, and there is a spectrum of findings
♦
Lymph nodes and spleen
Lymphoid hyperplasia is a common finding
There may be focal collections of macrophages or lympho-histiocytic aggregates that are discrete granulomas (similar to tubercles in appearance)
Partially calcified splenic granulomas have been observed many years after infection with Brucella suis
♦
Liver
Focal collections of macrophages may be present within portal triads
Nonnecrotizing granulomas that can be indistinguishable form sarcoid are described
Nonspecific hepatitis without granulomas also occurs
There may be chronic suppurative lesions with calcification
♦
Bone marrow
Granulomas in bone marrow have been noted in 75% of patients
•
These lesions tend to be small with indistinct borders
In addition, there may be increased macrophages
•
They may show erythrophagocytosis
♦
Other anatomic sites that may be involved less frequently include:
Heart and blood vessels (endocarditis, myocardial involvement, pericarditis, mycotic aneurysms)
Nervous system (meningitis, encephalitis, radiculoneuritis, cerebral mycotic aneurysm, peripheral neuropathy)
Bones and joints (sacroilitis, arthropathy of the hips, knees, or other joints; osteomyelitis of vertebrae and long bones)
Respiratory tract (pulmonary involvement is rare)
Gastrointestinal tract involvement due to Brucella melitensis has been described
Genitourinary tract involvement (chronic pyelonephritis, immune complex glomerulonephritis, salpingitis, and epididymoorchitis) has also been described
Special Stains
♦
These very small gram-negative coccoid rods are difficult to stain (e.g., with Brown–Hopps and other special stains) or identify in tissue sections
Diagnostic Techniques
♦
Cultures
When brucellosis is suspected, the microbiology laboratory must be notified before collection of specimens for culture
Specimens to collect for diagnosis include multiple blood cultures, bone marrow, and tissue from infected sites
Cultures require extended incubation times
•
Incubation of blood cultures for at least 2 weeks in automated systems
Serologic tests can also provide useful diagnostic results
•
In the USA, the serum agglutination test (SAT) is used widely
♦
Three species of Brucella, B. melitensis, B. suis, and B. abortus are considered Category A agents of bioterrorism in the USA
These are among the agents that pose the greatest threat relative to their infectious nature, ease of spread, or high mortality (e.g., Bacillus anthracis, Clostridium botulinum [botulinum toxins], Francisella tularensis, Yersinia pestis, variola major [smallpox], filoviruses [Marburg virus, Ebola virus], and arenaviruses [Lassa virus, Machupo virus])
♦
In a healthcare setting, laboratory procedures to be performed with Brucella species must be done in a facility with a biosafety level of at least 2
Activities that are at high risk for creating aerosols with this organism should be done in a biosafety level 3 laboratory
♦
If brucellosis is suspected in a clinical laboratory or sentinel laboratory, local and state public health authorities must be notified immediately
♦
The Laboratory Response Network toll-free HelpDesk ((866) 576–5227) is available for subject matter experts if discussions on individual organisms are needed
♦
Another excellent source of laboratory information relative to these types of agents is the American Society for Microbiology website (http://www.asm.org/index.php/guidelines/sentinel-guidelines)
Francisella tularensis and Tularemia
♦
F. tularensis is a very small (0.2 × 0.2–0.7 μm), nonmotile, strictly aerobic, catalase-positive, nonfermentative, gram-negative coccobacillus
♦
Virulent strains form a polysaccharide capsule that protects the organism from phagocytosis and from complement-mediated killing in serum
♦
F. tularensis, like Brucella sp., is a facultative intracellular pathogen that inhibits phagolysosomal fusion and replicates within macrophages
Epidemiology
♦
F. tularensis causes tularemia (also sometimes called rabbit fever, deer fly fever, or glandular fever)
♦
Naturally occurring tularemia is a zoonosis
It is worldwide (primarily northern hemisphere) in distribution
♦
An estimated 100–200 human cases of tularemia occur in the USA per year
♦
F. tularensis infects many mammals including various rodents, rabbits and hares (lagomorphs), squirrels, raccoons, cats, deer, and many other kinds of animals
Humans are considered accidental hosts
♦
It is naturally transmitted in several ways
Bite of infected tick, particularly including the dog tick (Dermacentor variabilis), wood tick (D. andersoni), and lone Star tick (Amblyomma americanum) in North America
Contact with an infected wild animal (e.g., rabbit hunter, muskrat trapper) or domestic animal [e.g., pet cat that caught infected mouse]
Inhalation of aerosols (e.g., by a squirrel hunter on a windy fall day)
Waterborne outbreaks (water contaminated by beavers or other animals)
♦
F. tularensis is hazardous for laboratory personnel
The infectious dose is very small
Clinical
♦
Symptoms may include headache, malaise, and fever/chills that usually begin abruptly 3–5 days after a tick bite or exposure to an infected animal
♦
Tularemia is subdivided into six forms
Ulceroglandular tularemia
•
Skin lesion at site of tick bite (in area of enlarged lymph node)
•
Reddish papule that ulcerates
•
Bacteremia and lymphadenopathy are also usually present
Glandular tularemia
•
Less common form
•
Enlarged, painful lymphadenopathy
•
No ulcerated skin lesion
Typhoidal tularemia
•
Also a less common form
•
Patient has sepsis clinically, but no lymphadenopathy or ulcerated skin lesion
•
Patient may have pneumonia or evidence of other organ involvement
•
Diagnosed usually by blood cultures
Oculoglandular tularemia
•
Rare; through direct contamination of conjunctiva (e.g., fingers; exposure to aerosols)
•
Ulcer on palpebral conjunctiva; enlargement of nearby lymph nodes
Oropharyngeal
•
It is acquired through ingestion of contaminated, poorly cooked meat
•
It also has been acquired from contaminated water
•
Manifestations include pharyngitis, tonsillitis, and cervical lymphadenopathy
♦
Pneumonic tularemia
•
Acquired by inhalation of aerosolized F. tularensis
•
Those at risk include hunters, farmers, sheep shearers, landscapers, and laboratory workers
•
Radiographic findings: bronchopneumonia (may be bilateral); or lobar infiltrates, hilar adenopathy, or pleural effusions; or focal infiltrates or apical infiltrates
Microscopic
♦
The inflammatory response includes the following features:
Acute: fibrin, neutrophils, macrophages, and T lymphocytes involved, often with liquefactive necrosis
Later: characterized by lymphocytes, macrophages, and giant cells
There also may be caseation, calcification, and fibrosis in chronic lesions
♦
Lymph nodes: reveal subcapsular or geographic areas of necrosis that may resemble infarcts
♦
Lungs: the findings may include extensive necrosis and cavitation
Special Stains
♦
F. tularensis is not visualized by use of any of the tissue gram stains
♦
Silver impregnation stains (e.g., Warthin–Starry, Steiner or Dieterle) may reveal tiny coccobacilli in macrophages
♦
Specific direct fluorescent antibody stains or immunohistochemical stains (formalin-fixed tissue) used at CDC
Diagnostic Techniques
♦
F. tularensis poses a biosafety hazard
The collection and processing of specimens are extremely hazardous for all involved
♦
It requires a sulfur-containing amino acid for growth (e.g., l-cysteine) and will grow slowly on buffered charcoal yeast extract (the BCYE agar that is used for isolation of Legionella) or on chocolate agar
It is not readily isolated on the usual unsupplemented sheep blood agar in a microbiology laboratory
♦
If tularemia is suspected clinically, the laboratory should be notified for the following reasons:
The high risk of transmission to laboratory workers exposed to this virulent pathogen necessitates special precautions be used for all specimen processing
•
Thus if tularemia is expected, specimen processing (using universal precautions) should be done under at least biosafety level [BSL] 2 conditions, and BSL-3 practices should be used if culture work is to be done
Prolonged incubation (i.e., 3–4 days to a week or longer) is required to isolate this slow-growing bacterium
♦
In addition, F. tularensis is considered a Category A agent of bioterrorism in the USA
It is listed among the agents that pose the greatest threat relative to their infectious nature, ease of spread, or high mortality (e.g., Bacillus anthracis, Brucella species Clostridium botulinum [botulinum toxins], Francisella tularensis, Yersinia pestis, variola major [smallpox], filoviruses [Marburg virus, Ebola virus], and arenaviruses [Lassa virus, Machupo virus])
♦
If F. tularensis is suspected in a clinical laboratory or sentinel laboratory, local and state public health authorities must be notified immediately
♦
The Laboratory Response Network toll-free HelpDesk ((866) 576–5227) is available for subject matter experts if discussions on individual organisms are needed
♦
Another excellent source of laboratory information relative to these types of agents is the American Society for Microbiology website (http://www.asm.org/index.php/guidelines/sentinel-guidelines)
Gram-Negative Cocci
Neisseria gonorrhoeae
♦
This is a gram -negative, oxidase and catalase-positive coccus (0.6 × 1 μm) that occurs as diplococci (pairs) with adjacent sides flattened
N. gonorrhoeae is second only to Chlamydia trachomatis as the most common bacterial cause of sexually transmitted disease in the USA
♦
Virulence factors of gonococci
Pili, PorB, and Opa outer surface proteins that mediate attachment to, and penetration of, epithelial cells
Lipopolysaccharide (endotoxin) of gonococci (GC), which stimulates secretion of tumor necrosis factor-alpha (TNF-α)
•
TNF-α (along with interleukin-1) is one of the major cytokines that mediates inflammation and the acute-phase responses associated with infection or injury (including the hemodynamic effects of septic shock)
•
TNF-α causes most of the symptoms of gonorrhea (e.g., damage to fallopian tubes)
A protease that cleaves IgA
Transferrin-, lactoferrin-, and hemoglobin-binding proteins that mediate the acquisition of iron for the metabolism of the organism
A β-lactamase that hydrolyses the β-lactam ring of penicillin
Clinical Manifestations
♦
Acute gonococcal urethritis: with dysuria, pyuria, and increased frequency of urination
♦
Cervicitis: neutrophilic, lymphocytic, and plasmacytic inflammation; focal epithelial necrosis and reactive cellular atypia
♦
Pelvic inflammatory disease; manifestations include endometritis, salpingitis, tuboovarian abscess (frequently involves mixed anaerobic and aerobic vaginal flora), pelvic peritonitis, and perihepatitis (Fitz-Hugh–Curtis syndrome) in varying combinations
♦
Rectal GC infection: ~40% of women with uncomplicated gonorrhea (also occurs in men who have sex with men)
♦
Bacteremia: occurs in ~0.5–3% of patients with gonorrhea
♦
Septic arthritis: in young adults; caused by N. gonorrhoeae relatively frequently. Usually presents as pain redness and swelling in a single joint
♦
Ophthalmia neonatorum (purulent conjunctivitis): the most common clinical manifestation in infants
♦
Pharyngitis
Microscopic
♦
Acute purulent inflammation
Granulation tissue and fibrosis (scar) occur later, particularly if the infection is not treated
♦
GC causes symptomatic urethritis, urethral stricture, and epididymitis in males
♦
GC causes acute inflammation with abscess formation in submucosal glands of females with subsequent spread to fallopian tubes leading to tuboovarian abscess (pelvic inflammatory disease), fibrosis, sterility, or ectopic pregnancy
Special Stains (Fig. 6.14)
♦
Gram stain is a sensitive and specific way to diagnose gonococcal urethritis in symptomatic (but not asymptomatic) males only
♦
The finding of neutrophils and intracellular gram-negative cocci in a direct smear of urethral exudate from males is considered diagnostic of gonorrhea
♦
For females, gram stain is not recommended (sensitivity and specificity are lacking)
♦
The gram stain appearance of Acinetobacter species in female genitourinary tract microbiota can be morphologically indistinguishable from that of N. gonorrhoeae
Fig. 6.14.
Neisseria gonorrhoeae; smear of exudate from male urethra (gram).
Diagnostic Techniques
♦
Culture, the historic “gold standard” diagnostic method, is sensitive and specific but has largely been replaced by amplified DNA probe tests (on discharge swab, urine, or Thin Prep)
♦
For testing children or persons who have been sexually assaulted for gonorrhoeae, culture is still considered the only appropriate means of diagnosis
Neisseria Meningitidis
♦
N. meningitidis is a gram-negative, oxidase and catalase-positive, encapsulated coccus that occurs as diplococci (pairs) with adjacent sides flattened
♦
It is commonly carried in the nasopharyngeal microbiota of healthy individuals
♦
Currently, there are 13 antigenic serogroups: A, B, C, D, X, Y, Z, E, W-135, H, I, K, and L
♦
Meningococcal disease occurs mainly when a new strain to which the person is not immune is encountered
♦
Transmission of a new strain of the organism (by respiratory droplets) is more likely to happen to young children or young adults living in crowded conditions (e.g., family members in the same household, soldiers in military barracks, college students in dormitories)
♦
In addition, the risk of developing meningitis is higher if the patient has a C5, C6, C7, or C8 (complement) deficiency
♦
Principal virulence factors of N. meningitidis include its polysaccharide capsule, pili, and lipopolysaccharide (LPS, which has endotoxin activity)
♦
Internalized into phagocytic vacuoles, the capsule protects the meningococcus against phagocytic killing
♦
The diffuse vascular damage that occurs in meningococcal infections is largely attributed to the LOS
Clinical Manifestations and Pathology
♦
Meningitis
N. meningitidis is the second most common cause of community-acquired meningitis in adults
The majority of cases are due to groups B and C
It occurs with or without sepsis
The manifestations include headache, meningeal signs, and fever
♦
Meningococcemia with sepsis
Occurs with or without meningitis (Fig. 6.15A)
Purpura fulminans (widespread hemorrhage)
Disseminated intravascular coagulation (DIC)
Waterhouse–Friderichsen syndrome (bilateral hemorrhagic necrosis of adrenals)
•
Is not unique to N. meningitidis
•
Caused also by H. influenzae
Microscopic (Fig. 6.15B, C)
♦
Acute purulent inflammation of the meninges can be prominent
♦
Gram stain reveals gram-negative diplococci
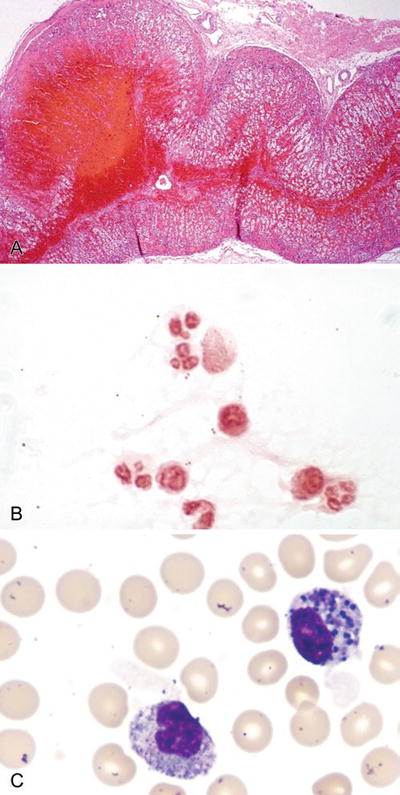
Fig. 6.15.
Adrenal hemorrhage and necrosis; (A) patient with meningococcal sepsis (Waterhouse–Friderichsen syndrome), (B) cerebrospinal fluid (gram), (C) peripheral blood buffy coat smear (Wright).
Diagnostic Techniques
♦
Gram stain of CSF is both sensitive and specific for N. meningitidis
However, other bacteria including Acinetobacter species can be morphologically similar
♦
Microbiologic culture is required for definitive diagnosis
Anaerobic Bacteria and Diseases
Anaerobic Sporeforming Rods: The Clostridia
♦
The genus Clostridium is composed of anaerobic, gram-positive, sporeforming rods
However , a few (e.g., C. tertium and C. histolyticum) are aerotolerant
♦
Unlike the genus Bacillus, Clostridium species typically form spores anaerobically but not aerobically
Clostridium species do not produce catalase
♦
Although there are ≥200 validly published species, only 30 or so are involved in diseases
Of these species, about half are toxigenic, capable of causing a number of important diseases (e.g., tetanus; pseudomembranous colitis)
♦
The principal habitats are soil and the intestinal tracts of humans and other animals
♦
Most of the time, the clostridia coexist peacefully within and around us, but circumstances may change leading to dire consequences (e.g., gas gangrene)
♦
Endogenous diseases involving clostridia from the host’s own microbiota are more common than exogenous diseases (e.g., food-borne botulism)
♦
Both toxin-mediated diseases (some with no or limited morphologic changes) and suppurative/necrotizing infections (with profound tissue damage) are produced
Clostridium Perfringens
♦
Clostridial myonecrosis (gas gangrene), more often than not, has a devastating outcome – with high morbidity and mortality
♦
C. perfringens type A is the most common cause
♦
Pathogenesis involves the following:
Traumatic injuries, both major and minor
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


