Metastatic Melanoma
Elizabeth A. Montgomery, MD
Key Facts
Terminology
Spread of malignant melanoma to gastrointestinal tract
In contrast to rare primary melanomas that typically involve esophagus and anus
Clinical Issues
Most common in small bowel (about 60%)
Poor overall prognosis
With medical treatment, mean survival 6 months
Some patients benefit from surgical debulking of metastases (mean survival 48 months )
Microscopic Pathology
“Bottom heavy” infiltrate of malignant cells
No in situ epithelial lesion
Pleomorphic cells with prominent nucleoli are classic
Some cases can have spindle cell phenotype
Often requires immunolabeling to confirm
S100, HMB-45, melan-A, MITF often used
Top Differential Diagnoses
Gastrointestinal stromal tumor
Gastrointestinal clear cell sarcoma
Langerhans cell histiocytosis
Schwannoma
Benign epithelioid nerve sheath tumor
PEComa
Carcinomas and lymphomas
Diagnostic Checklist
Always include metastatic melanoma in differential diagnosis of tumors in the GI tract
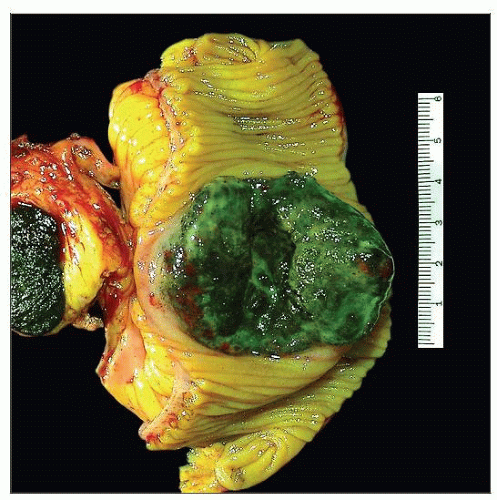 Gross photograph shows a specimen from a small bowel resection, taken from a patient with widely metastatic melanoma with resulting small bowel obstruction. Note the striking black pigmentation. |
TERMINOLOGY
Definitions
Spread of malignant melanoma to gastrointestinal tract
In contrast to rare primary melanomas that typically involve esophagus and anus
CLINICAL ISSUES
Site
Most common in small bowel (about 60%)
Colorectum (about 20%)
Stomach (about 10%)
Esophagus (about 5%)
Cases reported in gallbladder
Features of melanomas that metastasize to GI tract
Often in lower extremity
Often nodular
Presentation
Esophageal
Dysphagia, retrosternal pain, weight loss
Gastric
Upper GI tract bleeding, melena
Small bowel
Ileus
Colorectal
Abdominal pain, palpable mass, anemia, weight loss
Treatment
Drugs
Some responses to specific tyrosine kinase inhibitors such as imatinib
Subset has KIT mutations
Prognosis
Poor overall
With medical treatment, mean survival 6 months
Some patients benefit from surgical debulking of metastases (mean survival 48 months)
MICROSCOPIC PATHOLOGY
Key Descriptors
Histologic features
“Bottom heavy” infiltrate of malignant cells
No in situ epithelial lesion
Pleomorphic cells with prominent nucleoli classic
Intranuclear cytoplasmic pseudoinclusions
Some cases can have spindle cell phenotype
Mitotically active
Often requires immunolabeling to confirm
Most lesions lack classic black pigmentation
S100, HMB-45, melan-A, MITF often used
Pitfall: Many examples are CD117(+) although most examples are CD34(−)
DIFFERENTIAL DIAGNOSIS
Gastrointestinal Stromal Tumor
Most common in stomach muscularis propria
Uniform spindled or epithelioid cells
CD117(+), S100(−), CD34(+)
Gastrointestinal Clear Cell Sarcoma
Usually in ileum as primary neoplasms
Packeted arrangement of uniform cells
Large nucleoli
Have gene fusions: EWS-CREB1 or EWS-ATF1
Often S100(+) and negative with “specific” melanocytic markers
Molecular testing required to diagnose some cases
Langerhans Cell Histiocytosis
Schwannoma
Usually in stomach
Prominent lymphoid cuff
Spindle cells with lymphoplasmacytic backdrop
Minimal mitotic activity
No prominent nucleoli
S100(+); negative for “specific” melanoma markers
Benign Epithelioid Nerve Sheath Tumor
Usually in colon
Based in lamina propria and muscularis mucosae
Small epithelioid cells with prominent intranuclear pseudoinclusions
Essentially amitotic
Psammomatous Melanotic Schwannoma
Uniform small cells with delicate small nucleoli
Amitotic
Occasional overtly melanocytic cells
Psammoma bodies
S100(+), HMB-45(+), melan-A(+)
PEComa
Richly vascular lesions with epithelioid spindle cells
Includes family of neoplasms
Angiomyolipoma (essentially PEComa with fat), clear cell myomelanocytic tumor, lymphangioleiomyomatosis, and clear cell “sugar” tumor of lung
Unified by expression of smooth muscle markers that coexpress melanocytic markers
S100(−)
Carcinomas and Lymphomas
Generally separated using immunohistochemical panel
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



