Clinical Workup
Metastatic cancer to the skeleton is the most common malignant tumor affecting bone, the bone being the third most common site of metastasis after the lung and liver, with up to 85 percent of cancer patients at autopsy revealing bone metastasis. The true prevalence of bone metastasis is unknown, but it has been estimated that 350,000 people per year in the United States die with bone metastases (
1,
2). The most frequently seen primary tumor sites involving the skeleton in patients dying of metastatic cancer are breast, prostate, lung, thyroid, and kidneys (“paired organs”), which account for 80 percent of bone metastasis (
3). Whereas approximately 70 percent of patients with breast or prostate cancer develop bone metastases, the percentage in the case of those with lung and GI cancer is 20 to 30 (
4).
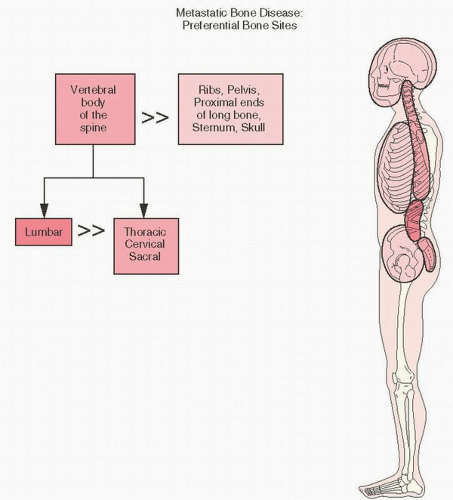
The most common sites of bone metastases are, in decreasing order of frequency, the thoracolumbar spine and sacrum, proximal femur, pelvis, ribs, sternum, proximal humerus, and calvarium.
Large autopsy series report a 30 to 70 percent rate of skeletal metastases with patients dying of malignant tumors, approximately 9 percent of which have a solitary skeletal metastasis (
5,
6); of these, roughly 5 to 10 percent have pathologic fractures due to bone metastasis (
Table 13.1).
A summary of autopsy studies compared the frequency of bone metastases from different primary sites (
7,
8,
9).
A review of several more recent autopsy studies comparing the frequency of metastases from different primary sites has not shown significant differences from Abram’s classic 1950 study cited in
Table 13.1 (
7,
8,
9).
The frequency and distribution of bone metastases in cancer has been explained by several factors, including anatomic and vascular features, as well as the bone microenvironment. Blood flow is high in the red marrow, which accounts for the predilection of metastases at red marrow sites (
Fig. 13.1). In addition, marrow stromal cells and bone matrix adhere to adhesive molecules produced by tumors. Bone itself contains many growth factors, which can be released when tumor cells destroy the bone. These factors, such as transforming growth factor β, insulin growth factors I and II, fibroblast growth factors, platelet-derived growth factors, and bone morphogenetic proteins, create an interactive and cascading microenvironment fertile for active promotion of further bone remodeling and further tissue factor release.
Interaction between the bone and its vasculature plays an additional important role by providing a source of mesenchymal stem cells including osteoprogenitors, an organizational structure, and a conduit for nutrients and angiogenic agents such as vasoactive endothelial growth factor (VEGF), a substance ubiquitous in cancers (
3). A paracrine endothelial-mesenchymal-histiocytic system has been proposed as a paradigm to explain the critical interaction of endothelial-derived substances with both bone cells and the histiocyte-related (osteoclastic) bone-resorbing factors, cells that dominate the osteolysis phenomenon of most cancers in bone.
Because metastatic cancer has a predilection for the marrow space of bone and, in particular, red marrow, metastases in children tend to involve long bones, whereas the axial skeleton is predilected in adults. As seen in osteomyelitis, a predisposition for the metaphyseal region has been hypothesized to be related to the hemodynamics of the bone vasculature, with increased sinusoidal pressure and slower blood flow promoting endothelial adhesion and subsequent extravasation of metastatic cells. In rare
circumstances, metastases may affect cortical bone, a phenomenon usually due to metastatic lung cancer. In general, the most commonly affected part of the skeleton in metastatic cancer to bone is the spine (
Fig. 13.1). The lumbar vertebrae are more commonly involved than the thoracic, cervical, and sacral vertebrae, with the exception of prostate cancer, which has a predilection for metastasis to the lumbar and sacral vertebral bodies, and breast and lung cancers, which prefer thoracic vertebrae (
3). Involvement of the posterior elements of the vertebrae by pathology is more likely to be due to tumor than infection. However, in the spine, malignant tumors more commonly involve the body of the vertebra, and benign tumors the posterior elements. Following the spine, the ribs, pelvis, proximal ends of the bones, sternum, and skull are involved by metastatic cancer in decreasing frequency.
Metastases distal to the elbow and knees (acral metastases) and to facial bones are unusual. Of acral metastases, those to the hand are three times more common than to the foot, affect more commonly the dominant hand, and are usually due to lung cancer. The most common site distal to the elbow and knee is the tibia (
5).
Metastases to the upper extremity are most commonly seen in the humerus (9.5 percent), scapula (5.7 percent), and clavicle (4.1 percent), with the radius, ulna, and hand each less than 1 percent (
6).
Clinically, bone metastases are associated with considerable morbidity, including pain, osteolysis, and pathologic fracture, spinal compression syndromes, hypercalcemia, and bone marrow replacement. Metastatic renal and thyroid tumors may have a significant increase in vascularity with even palpable pulses or bruits.
Pain is usually insidious in onset and of an aching character. As with osteoid osteoma, it is worse at night. Although poorly understood, factors related to pain include prostaglandins (as in osteoid osteoma and relieved similarly by prostaglandin-inhibiting drugs) and other chemical mediators (acetylcholine, histamine, bradykinin, serotonin). Osteolysis tumor-related cytokines, direct nerve infiltration, ion-channel stimulation, and local release of nerve growth factors are also implicated (
9).
In general, the clinical workup of a patient with a skeletal lesion of unknown etiology should include a careful and thorough history and physical exam. A battery of tests that should be considered are shown in Reference (
10).
Laboratory Findings
Although laboratory markers of metastatic cancer are, in general, nonspecific, laboratory and radiographic testing considerations for the workup of a new skeletal lesion have been proposed (
10) (
Table 13.2).
Alkaline phosphatase, a marker for the bone formation phase of bone turnover, is elevated in slightly more than 50 percent of cases. Serum glutamic-oxaloacetic transaminase (SGOT), lactate dehydrogenase (LDH), and uric acid levels are usually higher in patients with metastatic disease.
Laboratory tests that measure collagen moieties resulting from bone formation and resorption have been increasingly explored in the setting of metastatic disease (see
Chapter 3,
Fig. 3.13). The rate of collagen resorption as measured by serum levels of the n-telopeptide of type 1 collagen (NTX) is a strong predictor of skeletal complications and mortality (
9). Bone resorption can be demonstrated to be increased even in osteoblastic metastases, and the level of the serum marker NTX (n-telopeptide) is correlated with increased mortality in metastatic prostate carcinoma.
Osteoclasts resorbing bone release acid phosphatase but not in sufficient quantities to be clinically measurable and useful. Hypercalcemia, which may be indicative of bone lysis, is seen in up to 20 percent of patients. It is most common in patients with squamous carcinoma of lung, breast cancer, and renal carcinoma, as well as in myeloma and lymphoma. Osteolytic metastases are seen in 80 percent of such patients.
One clinically used triad in predicting bone metastases in the setting of bone pain is a serum LDH of more than 500 IU/L, a platelet count of less than 100,000, and a peripheral smear showing a leukoerythroblastic pattern of circulating erythroblasts and immature granulocytes.
Although there is not a single finding predictive of marrow infiltration, Papac (
11) found useful leukoerythroblastic changes in the peripheral blood smear, elevated LDH, and, to a lesser extent, cytopenias in the peripheral blood indicative of bone metastases. Leukoerythroblastic reactions have been attributed to tumor cells either mechanically crowding marrow cells or causing tumor cell-hematopoietic cytokine interaction.
Shinozaki et al. (
12) studied carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), carbohydrate antigens (CA 15-3, CA 19-9, and CA 125), and tissue polypeptide antigen (TPA) and found no diagnostically specific tests, but TPA, the most sensitive, was elevated in 61.5 percent of patients.
Imaging
Roentgenographically, conventional x-ray techniques lack sensitivity, with at least 40 percent of bone loss or lesions larger than 1.5 cm needed to show change on routine studies (
13,
14) (
Table 13.3).
However, radiography is a good modality for characterizing metastatic bone disease and has a role in assessing response to therapy. Healing usually results in progressive sclerosis. Routine radiographs can assess endosteal scalloping of the cortical bone and can be valuable in detecting early pathologic fracture. Since most metastases are primarily a process of bone lysis, osteosclerotic metastases can virtually exclude untreated tumors from sites such as the kidney and liver. But, never say never in medicine. Rarely, renal metastases are osteosclerotic (
15).
Roughly one-third of patients with cancer have overt metastases detected by physical examination and radiographic tests in their initial evaluation. The remaining two-thirds have no obvious metastases, but approximately 50 percent have occult microscopic disease. Metastatic cancer to the bone is characterized roentgenographically by many patterns including “geographic” (large well-defined lytic areas), “moth eaten” (smaller coalescing ill-defined areas), or “permeative” (tiny lytic defects).
Computed tomography (CT) scans and magnetic resonance imaging (MRI) studies may help define the extent of marrow and soft tissue involvement. Whereas CT better elaborates cortical bone involvement, MRI has advantages in detecting soft tissue and marrow extension and spinal column/cord disease. Bone metastases are usually seen as focal or diffuse hypointense dark lesions against a background of high signal (light) marrow on T1-weighted images.
Positron emission tomography (PET) scanning (18 fluorodeoxyglucose PET), based on the increased uptake of glucose in tumor cells, has been shown to be useful in the detection of certain tumors such as melanoma, lymphoma, non-small cell lung cancer, and carcinomas of unknown primary site (CUPS). The quantitative measurements used (so-called standardized uptake value [SUV]) are typically higher in malignant versus benign lesions.
Yamaguchi (
16) has outlined the usefulness of different imaging modalities for detecting vertebral metastases (
Table 13.3). A recent meta-analysis of studies comparing different imaging modalities for the detection of skeletal metastases shows that PET, PET/CT, and MRI had the highest sensitivity (approximately 90 percent) compared with CT and bone scintigraphy. The results of the pooled data sets are shown in
Table 13.3 (
17).
To detect most bone metastases, in particular, silent clinical metastasis, and attempt staging where appropriate, the bone scan is the most sensitive test (
Fig. 13.2).
In order for bone scans to be positive, there must be hypervascularity and/or new bone formation relative to adjacent normal host bone. Therefore, purely lytic metastases such as myeloma without pathologic fracture would be falsely negative. In these cases, skeletal surveys may be helpful. Although most metastatic tumors (and osteomyelitis) are osteolytic in bone, a mixed pattern of sclerosis and lysis is most often the case histologically, even if not detected on standard roentgenogram.
Roughly one-third of bone scans are positive before any radiologic evidence of metastatic tumor appears (
6). Scans are falsely negative in the setting of a positive roentgenogram in only 5 percent of cases. Whereas the main advantage of bone scanning is that it allows examination of the entire skeleton, the main disadvantage is lack of specificity.
Virtually all tumors cause some degree of osteolysis (
Fig. 13.3). Because the bone remodeling cycle of osteoclastic resorption and bone formation are linked, there is almost always some bone formation microscopically as well. Nonetheless, myeloma; many lymphomas; massive tumor metastases; and lung, kidney, thyroid, and gastrointestinal tumors are usually purely lytic. Breast may be lytic or mixed. Interestingly, purely blastic lesions are far less common and are accounted for mostly by prostate cancer (explaining the functional use of the bone scan in staging prostate cancer) and certain types of Hodgkin and carcinoid tumors.
Biopsy
Because roentgenographic findings in metastatic cancer are often nonspecific, biopsy is essential to establish the diagnosis. Criteria for biopsy vary, but should include at least those cases in which there is a lack of distinction from nonneoplastic disease. Because radiographs lack sensitivity, and bone scans specificity, biopsy is often essential. In one study of more than 1,000 patients with extraskeletal primary malignancies and solitary abnormalities on bone scan, 36 percent of the bone scan changes were due to a benign disorder (
17).
In performing percutaneous bone biopsies, the bone core is usually regarded as the prime biopsy specimen. Osseous blood that wells up through the biopsy or its sheath is frequently ignored, and there may be no special attempt to aspirate osseous blood. However, excellent morphologic structure can be seen in clots treated as tissue, that is, not smeared, but fixed in formalin, embedded in paraffin, and serially sectioned (
18). When comparing the diagnostic effectiveness of blood clot and smear, the blood clot can provide more diagnostic material, apparently because it contains a larger volume of blood than the smear. Both smear and blood clot are more satisfactory than the bone core in preserving tumor morphology because considerable crushing can be seen in the small bone
cores obtained with Craig or Ackerman trocars. Crushing artifact, together with some reactive bone formation, may interfere greatly with recognition of tumor morphology.
Another advantage of osseous blood aspiration is that it can be done with needles that are much smaller than the bone biopsy trocars. 17-gauge to 20-gauge needles are adequate, and thus biopsy may be safely done on cervical spine, upper dorsal spine, or rib, procedures that might be dangerous with larger trocars.
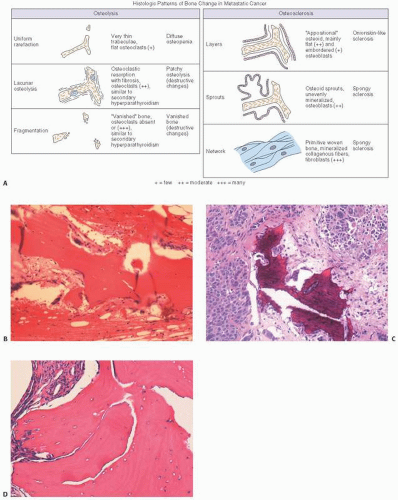
The cancellous bone may show significant alteration when involved by metastatic cancer, a phenomenon termed carcinomatous osteodysplasia (COD). Burkhardt et al. (
19) found such changes more commonly in adenocarcinomatous and scirrhous metastases, and divided the osseous reactions into several types (
Fig. 13.4).
Osteolysis was seen as (a) a diffuse osteopenia with attenuated trabeculae and (b) destructive resorption similar to that seen in hyperparathyroidism. Osteosclerosis was subdivided into three varieties: formation of primitive woven bone, juxtaposition of new bone on the original trabeculae (so-called creeping substitution), or a “sprouting” form in which branches of osteoid extended from the trabecular surface into the central marrow areas.
The most commonly encountered microscopic osseous reaction in metastatic cancer is a mixed picture of both osteolysis and osteosclerosis. Osteosclerosis alone is next most common, although it predominates in prostate cancer. Pure osteolysis is the least common (13 percent) unlike its prevalence observed roentgenographically, indicating that roentgenographic osteolysis masks a more commonly existing compensatory osteoblastic reaction noted microscopically. COD is useful in that tissue containing COD patterns may warn the clinician of a falsely negative procedure (i.e., biopsies where the actual cancer cells are not seen).
The Biology of Bone Metastatic Cancer
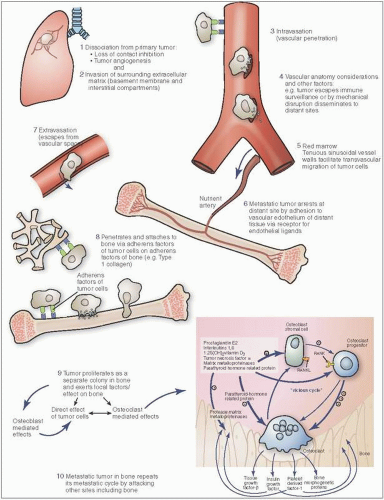
Ponder this, in order for metastatic bone cancer to occur, a series of extraordinary events must unfold (
Figs. 13.5 and
13.6). At the primary tumor site, the cancer cell, already in a heightened and proliferative metabolic state, has to develop growth factor independence, evade the normally programmed cell death (apoptosis), detach itself from the local primary cancer colony, and penetrate the layered endothelial cell web of a vascular wall (intravasation). It then has to survive the thunderous pulsation that constitutes blood flow and eventually escape the circulation after being flushed into the bone (extravasation). Now, in foreign hostile territory, it has to ward off or evade its potential destruction by the host immune response. If victorious, it needs to colonize the new site, create a supportive environment (angiogenesis), and reprogram its metabolism to survive the new territory even as it prepares to give birth to new cancer cells that will repeat the process. Amazing.
At least 10 specific “hallmark” features of this neoplastic process have been described (
20).
Sustained proliferation with growth factor independence The loss of regulation by growth-promoting and suppressing factors depends on mechanisms that include constitutive activation of replication-promoting pathways, autocrine stimulation by tumor cells producing their own growth factor ligands, and positive feedback loops with neighboring stromal cells that secrete growth factors for the tumor cells.
Evasion of proliferation suppressors
This occurs via regulators of cell cycle progression such as RB (retinoblastoma-associated gene) and TP-53, and the RB pathway that involves transduction of external signals. The TP53 pathway integrates intracellular signals including, but not limited to, DNA replicative damage.
It also occurs via loss of contact inhibition, including the common downregulation of E-cadherin (regulated by the NF-2 gene product “Merlin”), and the loss of function of the LKB-1 gene that maintains epithelial polarity.
Evasion and corruption of the TGF-β pathway also occurs. TGF-β normally functions as a suppressor of cell proliferation, but in neoplastic cells, it is reprogrammed to function in the metastasis-facilitating “epithelial-mesenchymal” transition (see below).
Resisting programmed cell death (apoptosis)
Corruption of the TP53-mediated DNA damage mechanism responds to serious DNA damage by activating apoptosis-promoting proteins. Autophagy and necrosis are also “programmed” modes of cell death.
Enabling “immortality.”
Somatic cells (excluding germ cells) are limited in the number of replicative cycles they can undergo. This is mediated largely by progressive shortening of telomeres (hexanucleotide repeats at the ends of chromosomes) with each cell division. The telomerase enzyme, which is activated in most neoplastic “immortalized” cells, counters this loss by adding back these short DNA segments.
Inducing angiogenesis
Angiogenesis in the postembryonic stage is limited to such states as wound healing and the menstrual cycle. Proliferating tumors require a vascular supply, which is mediated by angiogenesis-stimulating factors, most prominently VEGF-A (vascular endothelial growth factor A). VEGF-A expression can be induced by oncogenic signals, including Ras and Myc. Bone marrow-derived cells including macrophages, neutrophils, myeloid precursors, and mast cells also promote angiogenesis, and components of the tumor neovasculature may be derived from marrow precursor cells.
Activation of invasion and metastasis
This involves alteration of the relationship of the tumor cell to its environment (extracellular matrix and neighboring cells). Factors include loss of intercellular adhesion molecules such as E-cadherin, and upregulation of adhesion molecules involved in cell migration in embryogenesis such as N-cadherin. Establishment of metastatic disease is a multistep process involving local invasion, access to the vasculature, successful intravascular transit, extravasation, survival, and proliferation at the metastatic destination. Most of the steps of this process appear to be related to the “epithelial-mesenchymal transition,” a developmental program that (for epithelial neoplasms) leads to a mesenchymal phenotype that facilitates the aforementioned steps to metastasis. The acquisition of this phenotype is regulated by transcription factors including Snail, Slug, Twist, and Zeb1/2, which also have analogous functions in orchestrating cell motility in embryogenesis. According to this theory, the tumor cells undergo a reverse mesenchymal-to-epithelial transformation once established at the metastatic site. The initial steps in invasion are facilitated by stroma-degrading enzymes including MMPs (matrix metalloproteinases). Other, nonmesenchymal types of invasion, including collective and amoeboid, are also described.
Reprogramming metabolism
Normally, somatic cells utilize the aerobic Krebs cycle for glucose metabolism, favoring glycolysis under anaerobic conditions. Tumor cells may preferentially utilize glycolysis even under aerobic conditions compensating for the lower efficiency of anaerobic ATP synthesis by upregulating glucose transport into the cell. This may allow for more efficient synthesis of nucleosides and amino acids from by-products of the glycolytic pathway.
Evasion of immune destruction
The immune system recognizes and destroys many incipient tumors and metastases. This process is mediated primarily by cytotoxic T lymphocytes, T helper cells, and natural killer cells.
Of interest in this neoplastic process are the following:
Whereas only a small subset of cells in the primary tumor are capable of leading to bone metastasis, it has been estimated that up to 106 cells per gram of tumor may enter the bloodstream daily.
The vasculature wall is probably a homing site for some cells before they continue the passage.
It is only a limited number of primary tumor cells that eventually metastasize. It is estimated that less than 0.1 percent survive separation from a primary. Escape is facilitated by a complex array of events that includes the development of autonomous blood supplies, new hemodynamic forces involving tumor-induced fluid changes, and the expression of degradative enzymes such as matrix metalloproteinases.
For most types of tumors (excluding hematopoietic and some other nonepithelial neoplasms), the first step involves breaching the basement membrane and invading the extracellular matrix. Barriers to invasion include both cellular components such as the myoepithelial cells in the breast, and the mesothelial cells in the ovary. A further barrier to invasiveness is the tight intracellular junctions characteristic of epithelial linings, primarily mediated by E-cadherin. The epithelial-mesenchymal transition that leads to the loss of these junctions and loss of cell polarity confers a “mesenchymal” and invasive phenotype on the neoplastic cells. The basement membrane may be degraded by matrix metalloproteases, which have enhanced activity in neoplastic cells. Invasion of the local stroma frees growth factors sequestered in the extracellular matrix, as well as factors produced by stromal cells and local immune system cells, which further promote tumor growth and spread, including interleukin-6 (IL-6). Patterns of tumor invasion have been associated with differential expression of invasion-related genes in tumor cells. The local stromal reaction is similar to that seen in wound healing, and a positive feedback cycle between the invading tumor cells and the local stromal cells and environment has been characterized.
Vascular Invasion
Penetration into local lymphatic and, far more commonly with respect to bone, vascular channels is a critical early step. The neoangiogenesis described above as one of the “hallmarks” of neoplasia plays an important role in facilitating this. The new tumor vessels are leakier, less regular, and more continually remodeled than normal blood vessels. Substances such as type IV collagenase and heparinase may facilitate the burrowing into vessels. Other substances such as autocrine motility factors may facilitate this process. Tumor-associated macrophages (TAMSs) enhance intravasation of breast cancer cells by secreting epidermal growth factor (EGF), which stimulates the production of colony-stimulating factor-1 (CSF-1) by tumor cells, a macrophage stimulatory factor, leading to another positive feedback loop.
Following access to the circulation, spread by either venous (including retrograde flow via Batson’s plexus in prostate cancer) (
21) or arterial circulation occurs. Ordinarily, circulating tumor cells would undergo “anoikis,” which is a form of programmed cell death (apoptotic) secondary to loss of integrin-mediated adhesions to the extracellular matrix. Suppressors of the anoikis pathway have been identified in circulating tumor cells (CTCs). Circulating tumor cells are protected both from mechanical forces (hemodynamic shear) and from recognition and destruction by immune system cells by forming a protective coat consisting of a fibrin-platelet clot (
22).
Extravasation
Extravasation differs from intravasation due to the very different microenvironments of the two processes, including the different macrophage populations and the abnormal tumor neovasculature versus that at the metastatic site. In red marrow, vascular sinusoids are lined by endothelial cells, which lack a basement membrane and contain 60-angstrom fenestrae, features that facilitate tumor cell extravasation. Both gaps in endothelial cells and lack of basement membranes in sinusoidal vasculature probably facilitate this breach, leading to colonization. Molecules mediating adherence to endothelial structures include fibronectin, laminin, and collagen types IV and V (
22). The families of cell adhesion molecules are an ever-increasing list (
Table 13.4). Tumor cells may alter local vascular permeability. This has been described in the interaction of metastatic breast carcinoma cells in the lung, in which angiopoietin-like-4 (Angptl4) and other secreted factors including matrix metalloproteases promote hyperpermeability of the pulmonary vasculature. These mechanisms, however, are less active in bone metastasis.
Metastatic Growth
Arrival at the final metastatic site is not sufficient to ensure the development of clinically significant metastases. Indeed, it has been shown that most tumor cells that end up in distant sites either persist in a state of dormancy or undergo gradual cell death. Although the presence of micrometastases in the bone marrow of breast cancer patients is a significant predictor of cancer-specific survival, disease-free survival, and distant disease-free survival, their overall risk of distant disease in such patients was less than 50 percent (
23). The long-term persistence of subclinical metastases may be related to a balance between proliferation and cell death, the lack of a stimulatory microenvironment, or a failure to stimulate neoangiogenesis at the metastatic site.
Once arrived at the now potential metastatic cancer site, adherence/chemotactic factors and receptors on tumor cells and/or bone increase the likelihood of bone attachment. Chemoattractants include type I collagen peptides, proteoglycan, osteocalcin, synthetic peptides containing amino acids found in the collagen helix, and transforming growth factor β (
24). Stromal cells in bone are known
to produce factors that specifically stimulate growth of prostate cancer cells.