a. 1-reaction A, 2-reactions A and B to C, 3-reactions C to D and E
b. 1-reaction A, 2-reactions A and C to D, 3-reactions C to D and E
c. 1-reaction A, 2-reaction B to C, 3-reactions B to C and E
d. 1-reaction E, 2-reaction A, 3-reactions C to D and E
e. 1-reaction E, 2-reaction E, 3-reactions C to D and E
95. A 14-year-old African American adolescent competes well in short distance events as she tries out for the track team but does poorly at longer distances. Evaluation at a sports institute shows she has elevation of blood lactate after longer periods on a treadmill and suggests a defect in muscle energy metabolism. Although the girl’s parents decline further expensive evaluation, which of the following reactions of muscle metabolism would be least affected after she has run a long distance?
a. Glucose 6-phosphate to fructose 6-phosphate
b. Glucose to glucose 6-phosphate
c. Fructose 6-phosphate to fructose 1,6-diphosphate
d. Phosphoenolpyruvate to pyruvate
e. Pyruvate to lactate
96. Which of the following enzymes catalyzes high-energy phosphorylation of substrates during glycolysis?
a. Pyruvate kinase
b. Phosphoglycerate kinase
c. Triose phosphate isomerase
d. Aldolase
e. Glyceraldehyde-3-phosphate dehydrogenase
97. Which reaction in the figure below occurs in both muscle and liver but has substantially different qualities in the two?
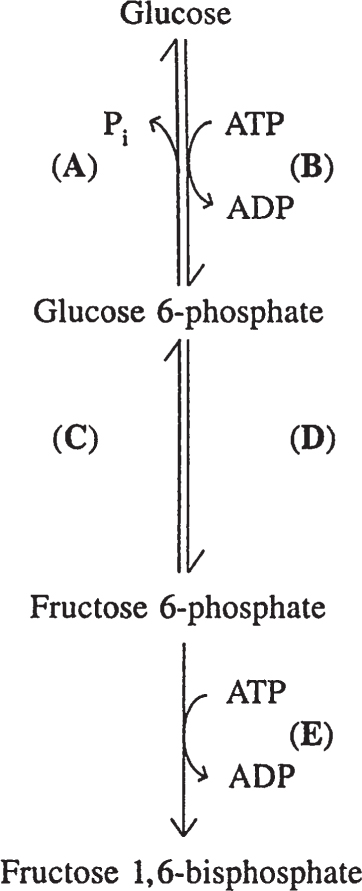
a. Reaction A
b. Reaction B
c. Reaction C
d. Reaction D
e. Reaction E
Carbohydrate Metabolism
Answers
66. The answer is a. (Murray, pp 132-139. Scriver, pp 1489-1520.) Glycosides are formed by condensation of the aldehyde or ketone group of a carbohydrate with a hydroxyl group of another compound. Other linked groups (aglycones) include steroids with hydroxyl groups (eg, cardiac glycosides such as digitalis or ouabain) or other chemicals (eg, antibiotics such as streptomycin). Sucrose (α-D-glucose-β-1 → 2-D-fructose), maltose (α-D-glucose-α-1 → 4-D-glucose), and lactose (α-D-galactose-β-1 → 4-D-glucose) are important disaccharides. Fructose is among several carbohydrate groups known as ketoses because it possesses a ketone group. The ketone group is at carbon 2 in fructose, and its alcohol group at carbon 1 (also at carbon 6) allows ketal formation to produce pyranose and furanose rings as with glucose. Most of the fructose found in the diet of North Americans is derived from the disaccharide sucrose (common table sugar). Sucrose is cleaved into equimolar amounts of glucose and fructose in the small intestine by the action of the pancreatic enzyme sucrase. Deficiency of sucrase can also cause chronic diarrhea. Hereditary fructose intolerance (MIM*229600) is caused by deficiency of the liver enzyme aldolase B, which hydrolyzes fructose 1-phosphate.
67. The answer is c. (Murray, pp 132-139. Scriver, pp 1521-1552.) Cellulose, the most abundant compound known, is the structural fiber of plants and bacterial walls. It is a polysaccharide, consisting of chains of glucose residues linked by β1 → 4 bonds. Since humans do not have intestinal hydrolases that attack β1 → 4 linkages, cellulose cannot be digested but forms an important source of “bulk” in the diet. Lactose is a disaccharide of glucose and galactose found in milk. Amylose is an unbranched polymer of glucose residues in β1 → 4 linkages. Glycogen is a branched polymer of glucose with both β1 → 4 and β1 → 6 linkages. Maltose is a disaccharide of glucose, which is usually the breakdown product of amylose.
68. The answer is b. (Murray, pp 237-249. Scriver, pp 1521-1552.) In humans, ethanol is cleared from the body by oxidation catalyzed by two NAD+-linked enzymes: alcohol dehydrogenase and acetaldehyde dehydrogenase (eliminating answers a, c-e). These enzymes act mainly in the liver to convert alcohol to acetaldehyde and acetate, respectively. In chronic alcoholics, alcohol dehydrogenase may be elevated somewhat. The NADH level is significantly increased in the liver during oxidation of alcohol, owing to the consumption of NAD+. This leads to a swamping of the normal means of regenerating NAD+. Thus, NAD+ becomes the rate-limiting factor in oxidation of excess alcohol.
69. The answer is d. (Murray, pp 660-675. Scriver, pp 5559-5586.) The exposure of tissues to chronic hypoxia makes them rely more on anaerobic metabolism for the generation of energy as ATP and other high-energy phosphates. Most tissues except for red blood cells can metabolize glucose under anaerobic or aerobic conditions (red blood cells do not have mitochondria for electron transport and must rely on other tissues to generate glucose back from lactate). In most tissues, a switch from aerobic to anaerobic metabolism greatly increases glucose utilization and decreases energy production. (Increased glucose utilization under anaerobic conditions in bacteria is known as the Pasteur effect after its discoverer.) Under aerobic conditions, the cell can produce a net gain, in moles of ATP formed per mole of glucose utilized that can be as high as 18 times that produced under anaerobic conditions. Thus, the cell generates more energy and requires less glucose under aerobic conditions. Such increased ATP concentrations, together with the release of citrate from the citric acid cycle under aerobic conditions, allosterically inhibit the key regulatory enzyme of the glycolytic pathway, phosphofructokinase. Decreased phosphofructokinase activity decreases metabolism of glucose by glycolysis.
70. The answer is c. (Murray, pp 237-249. Scriver, pp 1521-1552.) The principal pathway for hepatic metabolism of ethanol is thought to be oxidation to acetaldehyde in the cytoplasm by alcohol dehydrogenase. Acetaldehyde is then oxidized, chiefly by acetaldehyde dehydrogenase within the mitochondrion, to yield acetate. Acetone, methanol, hydrogen peroxide, and glycerol do not appear in this biodegradation pathway, eliminating answers a-b, d-e. The genetic variations of acetaldehyde dehydrogenase have few phenotypic effects aside from sensitivity to alcoholic beverages and are extremely common in the affected populations. These characteristics qualify acetaldehyde dehydrogenase variation as an example of enzyme polymorphism. Alcohol sensitivity is less of a factor in alcohol overdose or poisoning, where large intake overwhelms the liver’s capacity to metabolize about ounce per hour. Symptoms of coma and impending respiratory arrest mandate emergency room treatment to support respiration, avoid aspiration, reverse hypoglycemia (with IV dextrose), and restore depleted thiamine.
71. The answer is b. (Murray, pp 178-186. Scriver, pp 1521-1551.) Glycolysis in muscle produces lactate, which must be converted to glucose by liver or kidney via the Cori cycle (incorrect answers a, c, and e). Defects in liver glycogen metabolism therefore impair glucose 6-phosphate production or gluconeogenesis (alanine is also a substrate for gluconeogenesis—incorrect answer d) with resulting hypoglycemia and liver glycogen storage with or without toxicity (cirrhosis). Defects in muscle glycogen metabolism impair contraction (cramps, fatigue) with decreased serum lactate production during exercise and muscle glycogen accumulation (progressive weakness and atrophy).
Glucose 1-phosphate is the first intermediate in the conversion of glycogen to glucose. The enzyme glycogen phosphorylase catalyzes this first step. The second intermediate, glucose 6-phosphate, is subsequently converted to glucose by the enzyme glucose-6-phosphatase. This enzyme is found only in the liver and kidney; thus, these are the only tissues able to break down glycogen for use by other tissues. In tissues such as muscle, glycogen can be broken down to glucose 6-phosphate but can only be used in the cell in which it was produced.
72. The answer is d. (Scriver, pp 4517-4554. Murray, pp 197-206.) Glucose-6-phosphate dehydrogenase (G6PD) is the first enzyme of the pentose phosphate pathway, a pathway that metabolizes glucose to produce ribose and NADPH (eliminating answers a-c, e). Its deficiency (MIM*305900) is the most common enzymopathy, affecting 400 million people worldwide. It contrasts with glycolysis in its use of NADP rather than NAD for oxidation, its production of carbon dioxide, its production of pentoses (ribose, ribulose, xylulose), and its production of the high-energy compound PRPP (5-phosphoribosyl-1-pyrophosphate) rather than ATP. Production of NADPH by the pentose phosphate pathway is crucial for reduction of glutathione, which in turn removes hydrogen peroxide via glutathione peroxidase. Erythrocytes are particularly susceptible to hydrogen peroxide accumulation, which oxidizes red cell membranes and produces hemolysis. Stresses such as newborn adjustment, infection, or certain drugs can increase red cell hemolysis in G6PD-deficient individuals, leading to severe anemia, jaundice, plugging of renal tubules with released hemoglobin, renal failure, heart failure, and death. Since the locus encoding G6PD is on the X chromosome, the deficiency exhibits X-linked recessive inheritance with severe affliction in males and transmission through asymptomatic female carriers. Ribose 5-phosphate produced by the pentose phosphate pathway is an important precursor for ribonucleotide synthesis, but alternative routes from fructose 6-phosphate allow ribose synthesis in tissues without the complete cohort of pentose phosphate enzymes or with G6PD deficiency. The complete pentose phosphate pathway is active in liver, adipose tissue, adrenal cortex, thyroid, erythrocytes, testis, and lactating mammary gland. Skeletal muscle has only low levels of some of the enzymes of the pathway but is still able to synthesize ribose through fructose 6-phosphate.
73. The answer is c. (Murray, pp 197-206. Scriver, pp 1489-1520.) A special pathway for fructose metabolism (a specific fructokinase plus aldolase B and triokinase) is present in liver, kidney, and small intestine (incorrect answers a, b, d, and e). Foods high in sucrose (glucose-fructose) such as syrups, beverages, or diabetic substitutes yield high concentrations of fructose in the portal vein. Fructose is catabolized more rapidly than glucose by its specific fructokinase, bypassing hexokinase that is regulated by fasting and insulin. While providing a fuel for glycolysis, fructose also increases fatty acid, VLDL, and cholesterol-LDL production that are also side effects of diabetes mellitus due to the necessary shift to fat oxidation when intracellular glucose is less available. Recent data also suggest that fructose in soft drinks and other foods promotes insulin resistance and type II diabetes. For these reasons, the American Diabetic Association (www.diabetes.org) now suggests avoidance of fructose with the exception of naturally occurring fructose in fruits.
Fructose metabolism begins when fructokinase catalyzes the phosphorylation of fructose to fructose 1-phosphate, which is then split to D-glyceraldehyde and dihydroxyacetone by aldolase B. Triokinase converts D-glyceraldehyde to glyceraldehyde 3-phosphate, which can be metabolized further by glycolysis or be condensed with dihydroxyacetone phosphate by adolase to form fructose 1,6-diphosphate, glucose 6-phosphate, and glucose through gluconeogenesis.
74. The answer is b. (Murray, pp 178-186. Scriver, pp 1521-1552.) Glycogen synthesis and breakdown (glycogenolysis) are accomplished by separate pathways rather than reversible reactions. Glycogen synthase is active when dephosphorylated; glycogen phosphorylase is active when phosphorylated by a cyclic AMP-dependent protein kinase (incorrect answers a, c-e). These enzyme phosphorylations and dephosphorylations integrate glycogen synthesis/breakdown with food and glucose availability (refer to Fig. 8 in the High-Yield Facts).
Glycogen storage diseases are a group of inherited enzyme deficiencies that cause accumulation of glycogen in liver, heart, or muscle. Glucose is the primary source of energy for most cells and excess glucose is stored as glycogen. Glycogen provides for short-term high-energy consumption in muscle and is an emergency energy supply for the brain. Glycogen stored in the liver can be converted back to glucose for release into the blood stream for use by other tissues. Deficiency of adenylyl kinase or cAMP-dependent protein kinase can alter glycogenesis/glycogenolysis regulation and produce glycogen storage (refer to Table 3, High-Yield Facts).
75. The answer is a. (Murray, pp 178-186. Scriver, pp 1521-1552.) Normal glycogen is composed of glucose residues joined in straight chains by β1 → 4 linkages. At 4- to 10-residue intervals, a branch of β1 → 4 linkages is initiated at a β1 → 6 linkage (incorrect answers b-e). Glycogen particles can contain up to 60,000 glucose residues. In the absence of the debrancher enzyme, glycogen can be degraded only to the branch points, inhibiting release of glucose into the serum and causing glycogen storage. As noted in High-Yield Facts, Table 3, Forbes/Cori or type 3 glycogen storage disease (MIM*232400) involves deficiency of debranching enzyme.
76. The answer is e. (Murray, pp 178-186. Scriver, pp 1489-1520.) It is important to differentiate glucosuria due to diabetes mellitus or renal tubular problems from other sugars in the urine, like galactose in galactosemia or fructose/xylulose in essential fructosuria (all these are reducing sugars that are positive with Clinitest but only glucose is positive with the glucose oxidase reagent strip Dextrostix—the test is nonspecific like dipstick tests for hemoglobin/myoglobin). The uronic acid pathway, like the pentose phosphate pathway, provides an alternate fate for glucose without generating ATP. Glucose 6-phosphate is converted to glucose 1-phosphate and reacted with UTP to form the higher energy compound UDP-glucose. UDP-glucose is converted to UDP-glucuronic acid that is a precursor for glucuronide units in proteoglycan polymers. Unused glucuronic acid is converted to xylulose (incorrect answers a-d) and then to xylitol by a xylulose reductase, the enzyme deficiency in essential pentosuria (MIM*260800). In this “disease,” which is better called a trait, excess xylulose is excreted into urine but causes no pathology. Pentoses (5-carbon sugars) are important in the pentose phosphate and uronic acid pathways, providing ribose for nucleic acid metabolism. The other sugars listed as options except for lactose are 6-carbon hexoses.
77. The answer is a. (Murray, pp 132-139.) 6-Carbon hexoses with a C1 aldehyde group and four asymmetric carbons can generate 16 isomeric forms including ketoses with a C2 ketone group (eg, fructose—incorrect answers b-e) and aldoses with a C1 aldehyde group (glucose, galactose, and mannose). All are reducing sugars that will give a positive reducing substance reaction by urine dipstick, including fructose from the sucrose in table sugar. Glucofuranose and glucopyranose are ring structures of glucose, with the majority of glucose in solution in the glucopyranose form.
78. The answer is a. (Murray, pp 132-139. Scriver, pp 1521-1552.) Fructose is taken in by humans as sucrose, sucrose-containing syrups, and the free sugar. In liver, fructose is phosphorylated to fructose 1-phosphate by liver fructokinase, allowing it to bypass the ATP-regulated phosphofructokinase, yield glyceraldehyde and dihydroxyacetone phosphate by aldolase cleavage, and increase triglyceride/lipid biosynthesis (incorrect answers b-d). Fructose phosphorylation can also deplete liver cell ATP, lessening its inhibition of adenine nucleotide degradation and increasing uric acid that is the problem in gout. In adipocytes, fructose can be alternatively phosphorylated by hexokinase to fructose 6-phosphate. However, this reaction is competitively inhibited by appreciable amounts of glucose, as it is in other tissues.
79. The answer is c. (Murray, pp 132-139.) Glucose (glucopyranose) residues in cellulose are linked by β1 → 4 bonds in straight chains that humans cannot hydrolyze because they do not possess an enzyme to carry out this function. Cellulose is a structural constituent of plants that is insoluble and provides a source of fiber in the diet. Humans do have intestinal lactase to cleave galactose β1 → 4 glucose (lactose) bonds, maltase to cleave glucose β1 → 4 glucose (maltose) bonds, and sucrose to cleave glucose β1 → 4 fructose (sucrose) bonds. N-acetylcysteamine therapy must be started within 8 hours of acetaminophen ingestion to prevent and within 16 hours to ameliorate liver toxicity, a severe potentially lethal result if over 10 times the therapeutic dose of 5 mg/kg acetaminophen is ingested.
80. The answer is a. (Murray, pp 170-177. Scriver, pp 1471-1488.) Glucokinase promotes uptake of large amounts of glucose by the liver while the other enzymes are present in pathways active after glucose uptake (incorrect answers b-e). At normal glucose levels, the liver produces glucose from glycogen, but as glucose levels rise after feeding, the liver stops converting glycogen and instead takes up glucose. Insulin also plays a role in regulating blood glucose levels. Pancreatic β-cells produce insulin in response to hyperglycemia. Glucose uptake by the β-cells and phosphorylation by glucokinase stimulate secretion of insulin, which enhances glucose transport into adipose tissues and muscle and thus lowers blood glucose levels.
81. The answer is a. (Murray, pp 187-190. Scriver, pp 1521-1552.) Glucagon will stimulate gluconeogenesis and glycogenolysis in liver by increasing cAMP concentrations, thus raising blood glucose levels. The first step of gluconeogenesis is catalyzed by pyruvate carboxylase with consumption of carbon dioxide and utilization of one high-energy ATP phosphate bond (incorrect answers b-d):
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


