1 Metabolic response to injury, fluid and electrolyte balance and shock
The metabolic response to injury
Factors mediating the metabolic response to injury
The metabolic response is a complex interaction between many body systems.
The acute inflammatory response
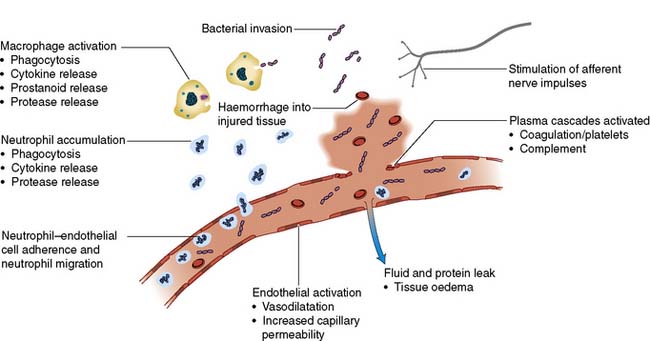
Inflammatory cells and cytokines are the principal mediators of the acute inflammatory response. Physical damage to tissues results in local activation of cells such as tissue macrophages which release a variety of cytokines (Table 1.1). Some of these, such as interleukin-8 (IL-8), attract large numbers of circulating macrophages and neutrophils to the site of injury. Others, such as tumour necrosis factor alpha (TNF-α), IL-1 and IL-6, activate these inflammatory cells, enabling them to clear dead tissue and kill bacteria. Although these cytokines are produced and act locally (paracrine action), their release into the circulation initiates some of the systemic features of the metabolic response, such as fever (IL-1) and the acute-phase protein response (IL-6, see below) (endocrine action). Other pro-inflammatory (prostaglandins, kinins, complement, proteases and free radicals) and anti-inflammatory substances such as antioxidants (e.g. glutathione, vitamins A and C), protease inhibitors (e.g. α2-macroglobulin) and IL-10 are also released (Fig. 1.1). The clinical condition of the patient depends on the extent to which the inflammation remains localized and the balance between these pro- and anti-inflammatory processes.
Table 1.1 Cytokines involved in the acute inflammatory response
| Cytokine | Relevant actions |
|---|---|
| TNF-α | Proinflammatory; release of leucocytes by bone marrow; activation of leucocytes and endothelial cells |
| IL-1 | Fever; T-cell and macrophage activation |
| IL-6 | Growth and differentiation of lymphocytes; activation of the acute-phase protein response |
| IL-8 | Chemotactic for neutrophils and T cells |
| IL-10 | Inhibits immune function |
(TNF = tumour necrosis factor; IL = interleukin)
The endothelium and blood vessels
The expression of adhesion molecules upon the endo-thelium leads to leucocyte adhesion and transmigration (Fig. 1.1). Increased local blood flow due to vasodilatation, secondary to the release of kinins, prostaglandins and nitric oxide, as well as increased capillary permeability increases the delivery of inflammatory cells, oxygen and nutrient substrates important for healing. Colloid particles (principally albumin) leak into injured tissues, resulting in oedema.
Afferent nerve impulses and sympathetic activation
1. Activation of the sympathetic nervous system leads to the release of noradrenaline from sympathetic nerve fibre endings and adrenaline from the adrenal medulla resulting in tachycardia, increased cardiac output, and changes in carbohydrate, fat and protein metabolism (see below). Interventions that reduce sympathetic stimulation, such as epidural or spinal anaesthesia, may attenuate these changes.
The endocrine response to surgery
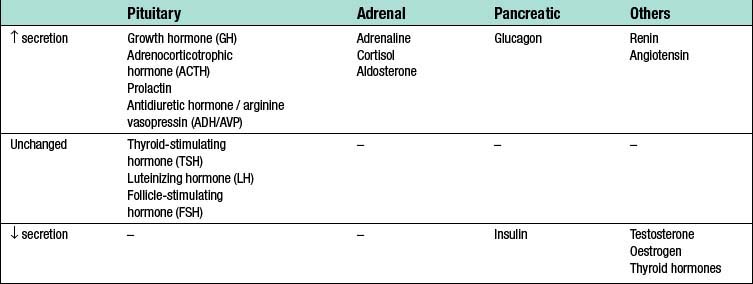
Surgery leads to complex changes in the endocrine mechanisms that maintain the body’s fluid balance and substrate metabolism, with changes occurring to the circulating concentrations of many hormones following injury (Table 1.2). This occurs either as a result of direct gland stimulation or because of changes in feedback mechanisms.
Consequences of the metabolic response to injury
Hypovolaemia
Reduced circulating volume often characterizes moderate to severe injury, and can occur for a number of reasons (Table 1.3):
• Loss of blood, electrolyte-containing fluid or water.
• Sequestration of protein-rich fluid into the interstitial space, traditionally termed “third space loss”, due to increased vascular permeability. This typically lasts 24–48 hours, with the extent (many litres) and duration (weeks or even months) of this loss greater following burns, infection, or ischaemia–reperfusion injury.
Table 1.3 Causes of fluid loss following surgery and trauma
| Nature of fluid | Mechanism | Contributing factors |
|---|---|---|
| Blood | Haemorrhage | Site and magnitude of tissue injury Poor surgical haemostasis Abnormal coagulation |
| Electrolyte-containing fluids | Vomiting | Anaesthesia/analgesia (e.g. opiates) Ileus |
| Nasogastric drainage | Ileus Gastric surgery | |
| Diarrhoea | Antibiotic-related infection Enteral feeding | |
| Sweating | Pyrexia | |
| Water | Evaporation | Prolonged exposure of viscera during surgery |
| Plasma-like fluid | Capillary leak/sequestration in tissues | Acute inflammatory response Infection Ischaemia–reperfusionsyndrome |
Summary Box 1.1 Factors mediating the metabolic response to injury
The acute inflammatory response
Inflammatory cells (macrophages, monocytes, neutrophils)
Proinflammatory cytokines and other inflammatory mediators
Decreased circulating volume will reduce oxygen and nutrient delivery and so increase healing and recovery times. The neuroendocrine responses to hypovolaemia attempt to restore normovolaemia and maintain perfusion to vital organs.
Fluid-conserving measures
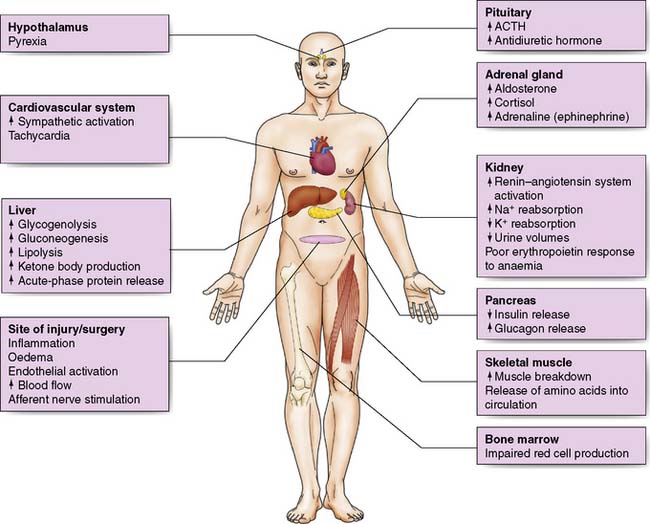
Oliguria, together with sodium and water retention – primarily due to the release of antidiuretic hormone (ADH) and aldosterone – is common after major surgery or injury and may persist even after normal circulating volume has been restored (Fig. 1.2).
Secretion of ADH from the posterior pituitary is increased in response to:
• Afferent nerve impulses from the site of injury
• Atrial stretch receptors (responding to reduced volume) and the aortic and carotid baroreceptors (responding to reduced pressure)
• Increased plasma osmolality (principally the result of an increase in sodium ions) detected by hypothalamic osmoreceptors
• Input from higher centres in the brain (responding to pain, emotion and anxiety).
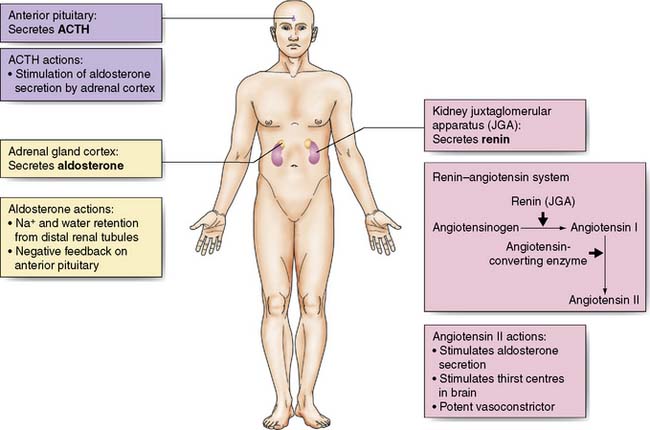
Aldosterone secretion from the adrenal cortex is increased by:
• Activation of the renin–angiotensin system. Renin is released from afferent arteriolar cells in the kidney in response to reduced blood pressure, tubuloglomerular feedback (signalling via the macula densa of the distal renal tubules in response to changes in electrolyte concentration) and activation of the renal sympathetic nerves. Renin converts circulating angiotensinogen to angiotensin (AT)-I. AT-I is converted by angiotensin-converting enzyme (ACE) in plasma and tissues (particularly the lung) to AT-II which causes arteriolar vasoconstriction and aldosterone secretion.
• Increased adrenocorticotropic hormone (ACTH) secretion by the anterior pituitary in response to hypovolaemia and hypotension via afferent nerve impulses from stretch receptors in the atria, aorta and carotid arteries. It is also raised by ADH.
• Direct stimulation of the adrenal cortex by hyponatraemia or hyperkalaemia.
Blood flow-conserving measures
Hypovolaemia reduces cardiac preload which leads to a fall in cardiac output and a decrease in blood flow to the tissues and organs. Increased sympathetic activity results in a compensatory increase in cardiac output, peripheral vasoconstriction and a rise in blood pressure. Together with intrinsic organ autoregulation, these mechanisms act to try to ensure adequate tissue perfusion (Fig. 1.3).
Increased energy metabolism and substrate cycling
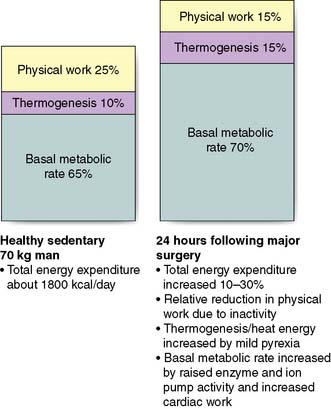
Although physical work usually decreases following surgery due to inactivity, overall energy expenditure may rise by 50% due to increased thermogenesis, fever and BMR (Fig. 1.4).
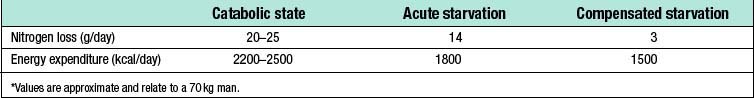
Catabolism and starvation
Catabolism
Protein metabolism
Skeletal muscle is broken down, releasing amino acids into the circulation. Amino acid metabolism is complex, but glucogenic amino acids (e.g. alanine, glycine and cysteine) can be utilized by the liver as a substrate for gluconeogenesis, producing glucose for re-export, while others are metabolized to pyruvate, acetyl CoA or intermediates in the Krebs cycle. Amino acids are also used in the liver as substrate for the ‘acute-phase protein response’. This response involves increased production of one group of proteins (positive acute-phase proteins) and decreased production of another (negative acute-phase proteins) (Table 1.4). The acute-phase response is mediated by pro-inflammatory cytokines (notably IL-1, IL-6 and TNF-α) and although its function is not fully understood, it is thought to play a central role in host defence and the promotion of healing.
Table 1.4 The acute-phase protein response
| Positive acute-phase proteins (↑ after injury) |
| Negative acute-phase proteins (↓ after injury) |
Changes in red blood cell synthesis and coagulation
• endothelial cell injury and activation with subsequent activation of coagulation cascades
• platelet activation in response to circulating mediators (e.g. adrenaline and cytokines)
• venous stasis secondary to dehydration and/or immobility
• increased concentrations of circulating procoagulant factors (e.g. fibrinogen)
• decreased concentrations of circulating anticoagulants (e.g. protein C).
Summary Box 1.3 Physiological changes in catabolism
Factors modifying the metabolic response to injury
The magnitude of the metabolic response to injury depends on a number of different factors (Table 1.6) and can be reduced through the use of minimally invasive techniques, prevention of bleeding and hypothermia, prevention and treatment of infection and the use of locoregional anaesthesia. Factors that may influence the magnitude of the metabolic response to surgery and injury are summarised in table 1.6.
Table 1.6 Factors associated with the magnitude of the metabolic response to injury
| Factor | Comment |
|---|---|
| Patient-related factors | |
| Genetic predisposition | Gene subtype for inflammatory mediators determines individual response to injury and/or infection |
| Coexisting disease | Cancer and/or pre-existing inflammatory disease may influence the metabolic response |
| Drug treatments | Anti-inflammatory or immunosuppressive therapy (e.g. steroids) may alter response |
| Nutritional status | Malnourished patients have impaired immune function and/or important substrate deficiencies. Malnutrition prior to surgery is associated with poor outcomes |
| Acute surgical/trauma-related factors | |
| Severity of injury | Greater tissue damage is associated with a greater metabolic response |
| Nature of injury | Some types of tissue injury cause a disproportionate metabolic response (e.g. major burns), |
| Ischaemia–reperfusion injury | Reperfusion of ischaemic tissues can trigger an injurious inflammatory cascade that further injures organs. |
| Temperature | Extreme hypo- and hyperthermia modulate the metabolic response |
| Infection | Infection is associated with an exaggerated response to injury. It can result in systemic inflammatory response syndrome (SIRS), sepsis or septic shock. |
| Anaesthetic techniques | The use of certain drugs, such as opioids, can reduce the release of stress hormones. Regional anaesthetic techniques (epidural or spinal anaesthesia) can reduce the release of cortisol, adrenaline and other hormones, but has little effect on cytokine responses |
Fluid and Electrolyte Balance
• ADH and aldosterone secretion as described above
• Loss from the gastrointestinal tract (e.g. bowel preparation, ileus, stomas, fistulas)
• Insensible losses (e.g. sweating secondary to fever)
• Third space losses as described above
• Medications (e.g. diuretics)
• Underlying chronic illness (e.g. cardiac failure, portal hypertension).
Normal water and electrolyte balance
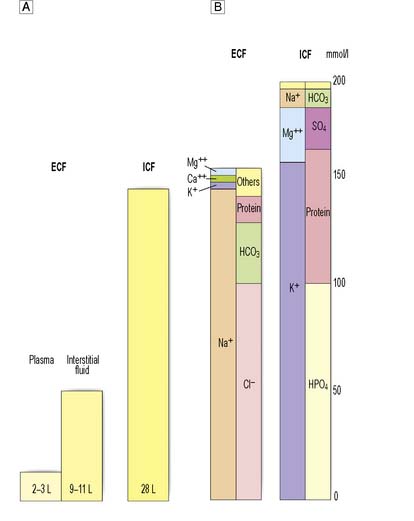
Water forms about 60% of total body weight in men and 55% in women. Approximately two-thirds is intracellular, one-third extracellular. Extracellular water is distributed between the plasma and the interstitial space (Fig. 1.5A).
The differential distribution of ions across cell membranes is essential for normal cellular function. The principal extracellular ions are sodium, chloride and bicarbonate, with the osmolality of extracellular fluid (normally 275–295 mOsmol/kg) determined primarily by sodium and chloride ion concentrations. The major intracellular ions are potassium, magnesium, phosphate and sulphate (Fig. 1.5B).
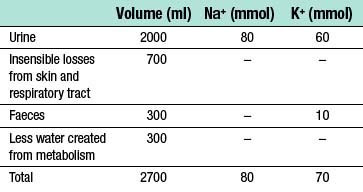
• 2500 to 3000 ml of fluid is lost via the kidneys, gastrointestinal tract and through evaporation from the skin and respiratory tract
• fluid losses are largely replaced through eating and drinking
• a further 200–300 ml of water is provided endogenously every 24 hours by the oxidation of carbohydrate and fat.
The daily requirement for both sodium and potassium in children is about 2–3 mmol/kg.
Assessing losses in the surgical patient
Only by accurately estimating (Table 1.8) and, where possible, directly measuring fluid and electrolyte losses can appropriate therapy be administered.
Table 1.8 Sources of fluid loss in surgical patients
| Typical losses per 24 hrs | Factors modifying volume | |
|---|---|---|
| Insensible losses | 700–2000 ml | ↑ Losses associated with pyrexia, sweating and use of non-humidified oxygen |
| Urine | 1000–2500 ml | ↓ With aldosterone and ADH secretion; ↑ With diuretic therapy |
| Gut | 300–1000 ml | ↑ Losses with obstruction, ileus, fistulae and diarrhoea (may increase substantially) |
| Third-space losses | 0–4000 ml | ↑ Losses with greater extent of surgery and tissue trauma |
The effect of surgery
Loss from the gastrointestinal tract
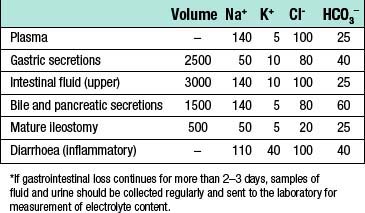
The magnitude and content of gastrointestinal fluid losses depends on the site of loss (Table 1.9):
• Intestinal obstruction. In general, the higher an obstruction occurs in the intestine, the greater the fluid loss because fluids secreted by the upper gastrointestinal tract fail to reach the absorptive areas of the distal jejunum and ileum.
• Paralytic ileus. This condition, in which propulsion in the small intestine ceases, has numerous causes. The commonest is probably handling of the bowel during surgery, which usually resolves within 1–2 days of the operation. Occasionally, paralytic ileus persists for longer, and in this case other causes should be sought and corrected if possible. During paralytic ileus the stomach should be decompressed using nasogastric tube drainage, and fluid losses monitored by measuring nasogastric aspirates.
• Intestinal fistula. As with obstruction, fistulae occurring high in the gut are associated with the greatest fluid and electrolyte losses. As well as volume, it may be useful to measure the electrolyte content of the fluid lost in order to determine the fluid replacement required.
• Diarrhoea. Patients may present with diarrhoea or develop it during the perioperative period. Fluid and electrolyte losses may be considerable.
Intravenous fluid administration
When choosing and administering intravenous fluids (Table 1.10) it is important to consider:
• what fluid deficiencies are present
• the fluid compartments requiring replacement
• any electrolyte disturbances present
Types of intravenous fluid
Crystalloids
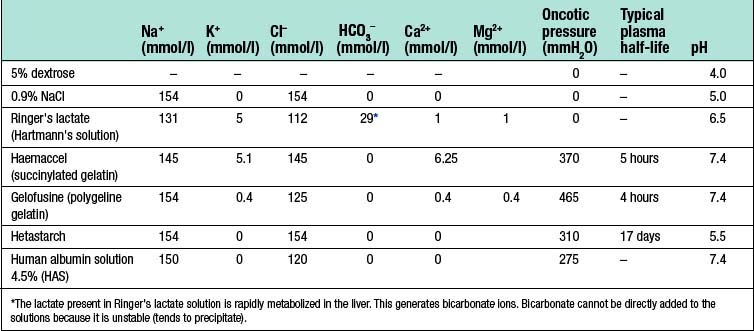
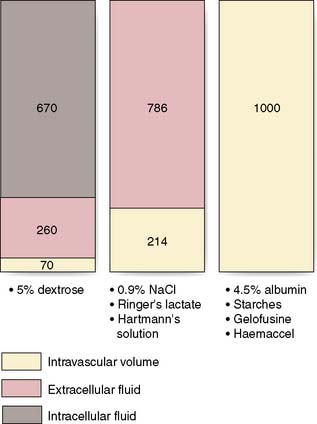
Dextrose 5% contains 5 g of dextrose (d-glucose) per 100 ml of water. This glucose is rapidly metabolized and the remaining free water distributes rapidly and evenly throughout the body’s fluid compartments. So, shortly after the intravenous administration of 1000 ml 5% dextrose solution, about 670 ml of water will be added to the intracellular fluid compartment (IFC) and about 330 ml of water to the extracellular fluid compartment (EFC), of which about 70 ml will be intravascular (Fig. 1.6). Dextrose solutions are therefore of little value as resuscitation fluids to expand intravascular volume. More concentrated dextrose solutions (10%, 20% and 50%) are available, but these solutions are irritant to veins and their use is largely limited to the management of diabetic patients or patients with hypoglycaemia.
Sodium chloride 0.9% and Hartmann’s solution are isotonic solutions of electrolytes in water. Sodium chloride 0.9% (also known as normal saline) contains 9 g of sodium chloride dissolved in 1000 ml of water; Hartmann’s solution (also known as Ringer’s lactate) has a more physiological composition, containing lactate, potassium and calcium in addition to sodium and chloride ions. Both normal saline and Hartmann’s solution have an osmolality similar to that of extracellular fluid (about 300 mOsm/l) and after intravenous administration they distribute rapidly throughout the ECF compartment (Fig. 1.6). Isotonic crystalloids are appropriate for correcting EFC losses (e.g. gastrointestinal tract or sweating) and for the initial resuscitation of intravascular volume, although only about 25% remains in the intravascular space after redistribution (often less than 30–60 minutes).
Colloids
The theoretical advantage of colloids over crystalloids is that, as they remain in the intravascular space for several hours, smaller volumes are required. However, overall, current evidence suggests that crystalloid and colloid are equally effective for the correction of hypovolaemia (EBM 1.1).