Metabolic Diseases of Bone
Deborah Novack
I. NORMAL ANATOMY. Because of its accessibility and composition of both cortical and trabecular (cancellous) bone, the iliac crest is the site of choice for evaluation of systemic metabolic bone diseases. Cortex forms the external layer of all bones, comprises ˜80% of bone mass, and supports most of the tissue’s mechanical function. Trabecular bone, the meshwork surrounded by marrow or fat, is much more metabolically active than cortex. To support both its mechanical and metabolic functions, bone is dynamically regulated, and the skeleton is replaced completely every 10 years. The process of replacement, known as remodeling or turnover, is accomplished by the coordinated action of bone-forming osteoblasts (OBs) and bone-resorbing osteoclasts (OCs), and is regulated by a variety of systemic factors including calcium, phosphorus, and parathyroid hormone (PTH). The goal of iliac crest trochar biopsy is to assess this process.
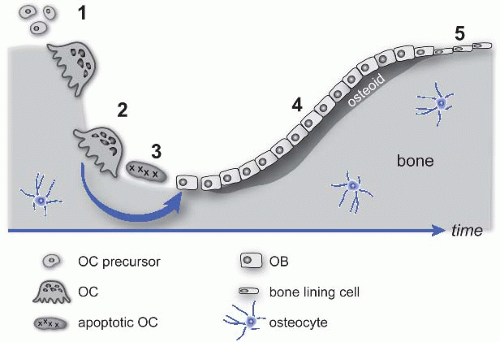
In either the cortex or the trabeculum, remodeling begins when mononuclear OC precursors (derived from hematopoietic progenitors) arrive at a bone surface, fuse, and differentiate into functional polykaryons (Fig. 49.1). Mature OCs polarize and secrete acid and proteases onto an isolated microenvironment of the bone surface, excavating a pit known as Howship’s lacuna. This resorption phase ends with the OCs’ apoptosis, and a reversal phase follows, characterized by activation of OBs (of mesenchymal origin) to replace the excavated bone; the activity of OCs and OBs is normally tightly coupled, and the amount of bone synthesized matches the amount resorbed. Newly secreted matrix called osteoid becomes mineralized to form mature bone. The remodeling cycle ends when new bone formation is complete, and the OBs are either incorporated into the new bone matrix as osteocytes or become quiescent surface bone lining cells. The net result of each cycle is the formation of a new osteon, a packet of bone delineated by a “cement line” in which the collagen fibers are aligned. These lamellae of bone are easily seen when decalcified hematoxylin and eosin (H&E)-stained sections are examined under polarized light (e-Fig. 49.1).*
OCs can be identified by their characteristic appearance as multinucleated cells on the bone surface, with discrete nuclei (in contrast to megakaryocytes, which have fused nuclei). The most sensitive method of identifying OCs histologically is expression of tartrate-resistant acid phosphatase (TRAP), which stains OCs bright red (e-Fig. 49.2), although this is not usually necessary for diagnosis. OBs appear as cuboidal cells on the bone surface, often in rows, with abundant cytoplasm and an eccentric nucleus (e-Fig. 49.3). However, the strength of the undecalcified biopsy is in the evaluation of the function of these cells rather than their morphology.
OBs secrete matrix proteins onto the bone surface, but several days are required for mineral deposition. Therefore, the extent of bone surface covered by osteoid (osteoid surface) is one indicator of OB activity. The thickness of the osteoid seams reflects the rate of mineral apposition, because mineralization converts osteoid to
bone. Decalcification required for standard paraffin processing removes the distinction between newly synthesized osteoid and mature calcified bone. In contrast, undecalcified plastic sections can be stained in several ways to demonstrate osteoid. The von Kossa stain, a silver-based stain used with a basic fuchsin counterstain, shows calcified bone matrix as dark brown or black, whereas the unmineralized matrix (osteoid) appears pink-red (e-Fig. 49.4A). A trichrome stain, either Goldner (e-Fig. 49.3) or modified Masson (e-Fig. 49.4B), also distinguishes mineralized bone from osteoid. These latter stains allow easier interpretation of cellular morphology than the von Kossa, and also highlight peritrabecular or marrow fibrosis.
A second critical marker of OB function is tetracycline labeling. Tetracycline family antibiotics are calcium-chelating fluorochromes that bind to actively mineralizing bone surfaces, can be taken orally, and are well-tolerated. They are given in two courses, separated by 2 weeks (see below). If bone formation is active during both intervals, examination of unstained sections by fluorescence microscopy demonstrates two bright bands of labeling (a double label) (e-Fig. 49.5). Similarly, active bone formation during only one of the labeling periods yields a single tetracycline label (e-Fig. 49.5). Combination of the extent of labeled trabecular bone surface and the distance between labels provides the mineral apposition rate and bone formation rate. In a normal subject, most surfaces with osteoid, as seen on trichrome or von Kossa stains, have single or double labels.
During normal endochondral bone development, cartilage formed at the growth plate is replaced by bone in the primary spongiosa through the action of OCs. Toluidine blue stains cartilage purple, and cartilage may be found within trabeculae near the growth plate in children (e-Fig. 49.6). However, a finding of entrapped
cartilage in an iliac crest bone biopsy in an adult, or >1 cm from the growth plate in a child, is indicative of OC dysfunction such as in osteopetrosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree