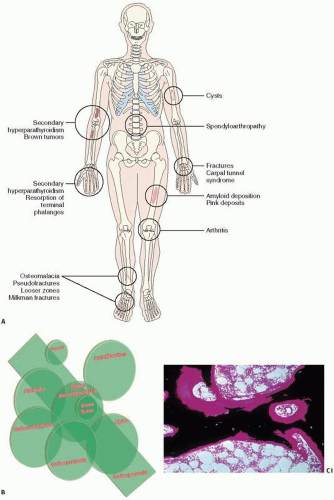
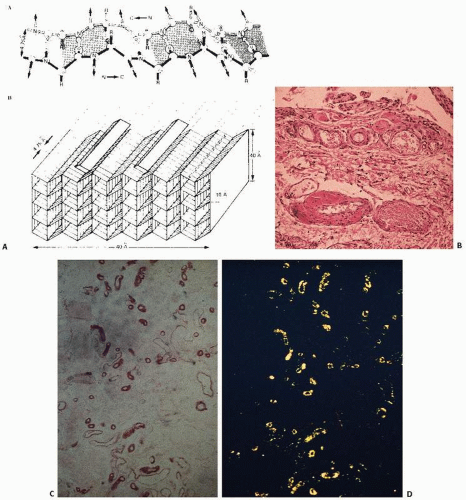
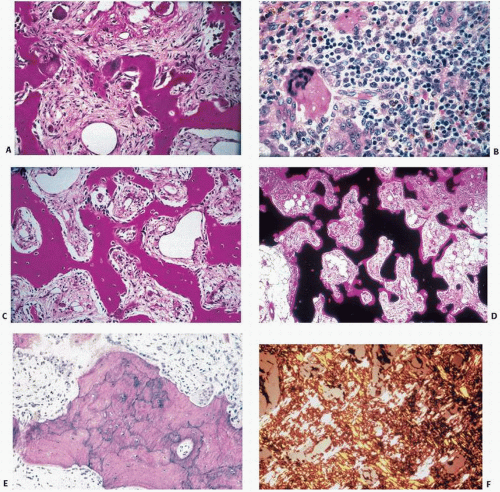
of bone biopsy specimens from large series of patients who have long-standing end-stage renal disease with or without hemodialysis has revealed a broader range of changes, which include secondary hyperparathyroidism, osteomalacia, or a mixture of osteomalacia and secondary hyperparathyroidism. Histomorphometric studies in which tetracycline labeling is used have also revealed a type of ROD characterized by sparse cellular activity and low bone turnover (aplastic bone) (6) (Table 4.1). In addition, tissue samples from these patients may or may not have detectable aluminum and/or iron deposition at the mineralization fronts. Rare circumstances of iron at the mineralization front causing osteomalacia have been described (7). There are also patients with an increasing range of amyloid-related syndromes resulting from the deposition of β2-microglobulin (8). An additional unusual tissue change, which is most evident on x-ray films, is osteosclerosis, a condition characterized by increased bone deposition in tissue despite obvious identifiable osteoclastic resorption.
TABLE 4.1 Histomorphometric and Histologic Classification of Renal Osteodystrophy | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
phosphate retention,
↓ calcitriol levels,
↓ serum ionized calcium,
↓ numbers of vitamin D receptors and ↓ numbers of calcium sensors in the parathyroid gland,
skeletal resistance to the calcemic action of PTH due to ↓ density of PTH receptors on osteoblasts; ↑ serum osteoprotegerin levels.
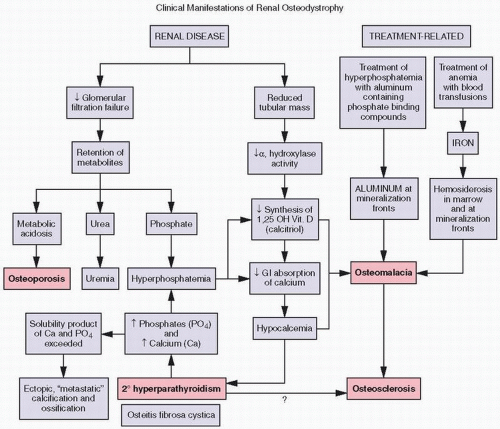 FIGURE 4.2. Biochemical (purple boxes) and osseous histologic (pink boxes) abnormalities in renal osteodystrophy. |
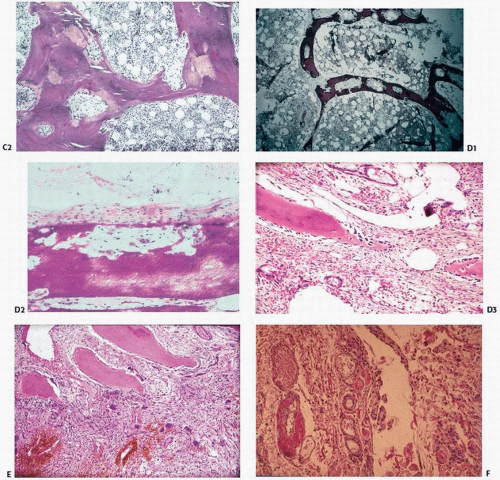
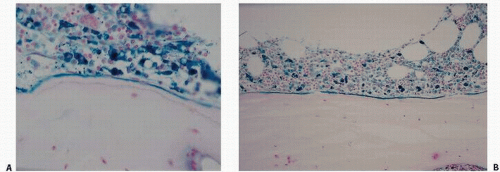
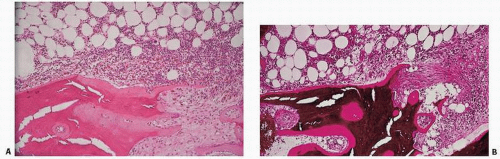
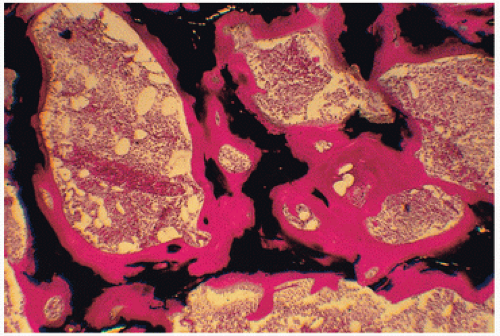
study of the uptake and activity of tetracycline at the bone surface, reveal at least five recognizable types of bone remodeling disease in ROD: (a) secondary hyperparathyroidism, (b) osteomalacia, (c) mixed uremic dystrophy (secondary hyperparathyroidism and osteomalacia), (d) adynamic or low-turnover disease, and (e) osteosclerosis (11). Stains for aluminum, iron, and amyloid should be performed, as all these may contribute to pathology.
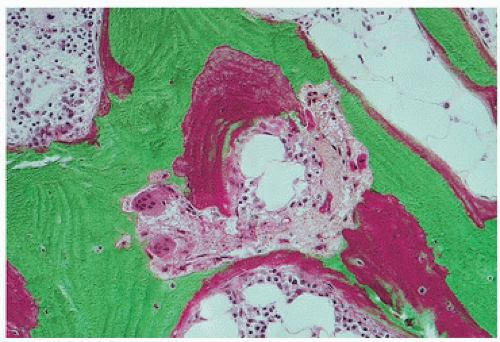
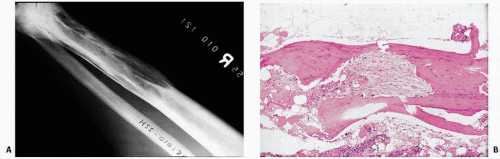
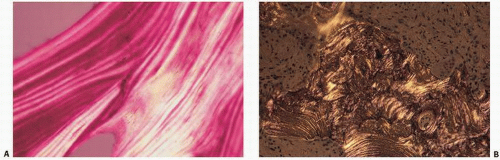
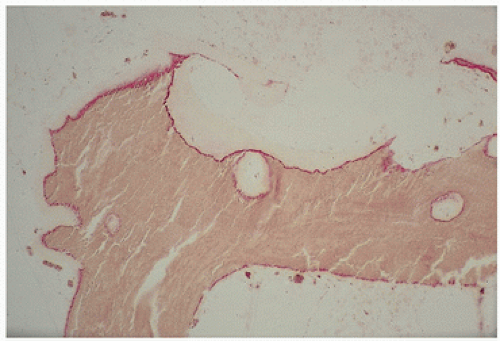 FIGURE 4.3. Aluminum (red) osteomalacia in renal-related bone disease develops at the osteoid-bone interface (“mineralization front”) and is directly associated with osteoid accumulation. |
multiple, “brown tumors” represent the disorder von Recklinghausen described as osteitis fibrosa cystica, a rare observation in modern times due to early diagnosis and treatment of hyperparathyroidism.
increase in systemic calcium load, will limit soft tissue calcification and potentially reduce cardiovascular risk.
appearance of amorphous, eosinophilic deposits under light microscopy after hematoxylin and eosin (H&E) staining,
bright green birefringence under polarized light after staining with the dye Congo red,
regular fibrillary structure observed with electron microscopy,
β-pleated sheet structure demonstrated with x-ray diffraction.
ocular and renal pathology are seen. Treatment is directed at reducing the amount of methionine (a dietary precursor of cystine) and cystine in the diet or using drugs that can reduce intracellular cystine levels. When possible, renal transplantation should be performed to bypass the genetically determined defect in the intracellular environment.
TABLE 4.2 Nomenclature and Classification of Human Amyloid and Amyloidosis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 4.3 Syndromes and Clinical Manifestations of β2-Microglobulin Amyloid Deposition | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
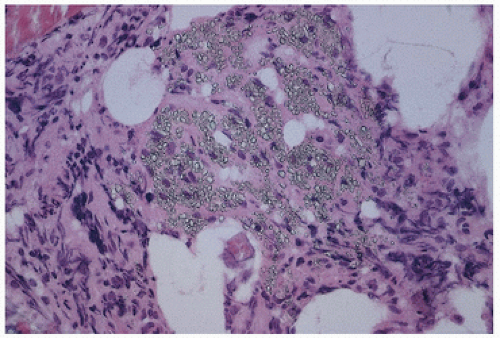
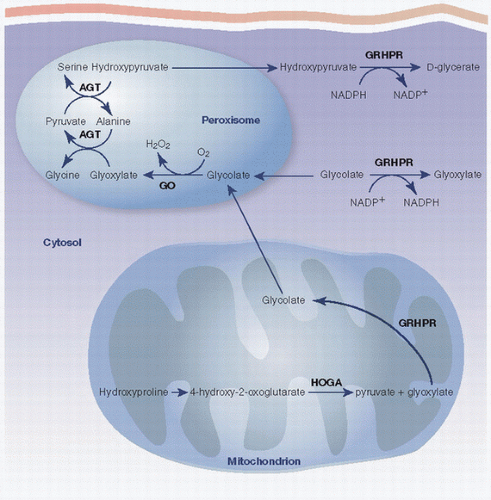
within membrane-limited organelles, having the appearance and enzyme markers of lysosomes, that is, containing acid phosphatase. The specific localization of crystals within lysosomes has suggested a classification of cystinosis as a lysosomal storage disease.
TABLE 4.4 Some Distinguishing Characteristics of the Three Major Types of Cystinosis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
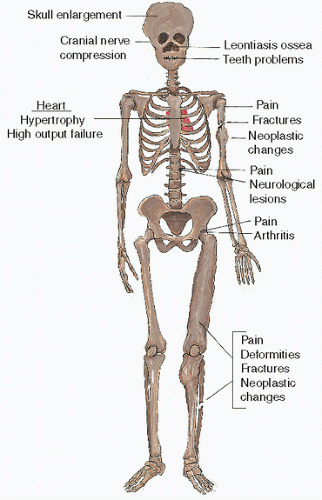
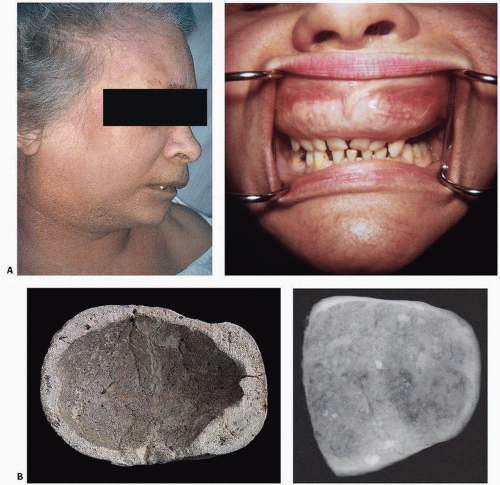
 FIGURE 4.14. Grotesque facial features are due to enlarged frontal skull bone and maxilla. (After Quinten Metsys [1465/66-1530], The National Gallery, London.) |
TABLE 4.5 Symptoms of Paget’s Disease | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
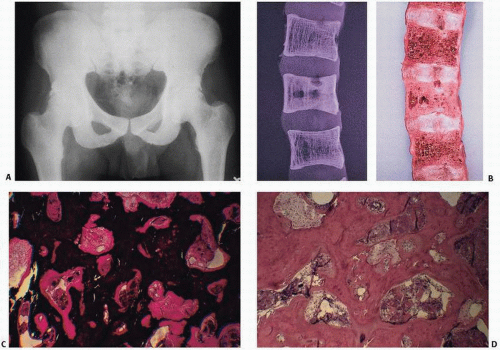
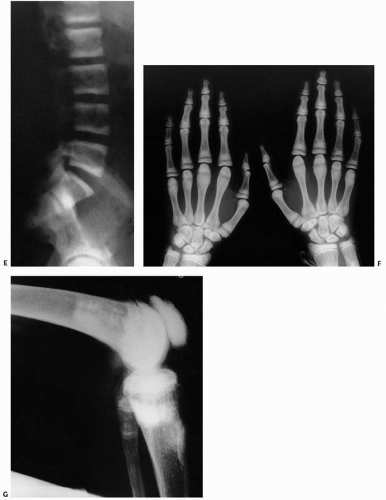
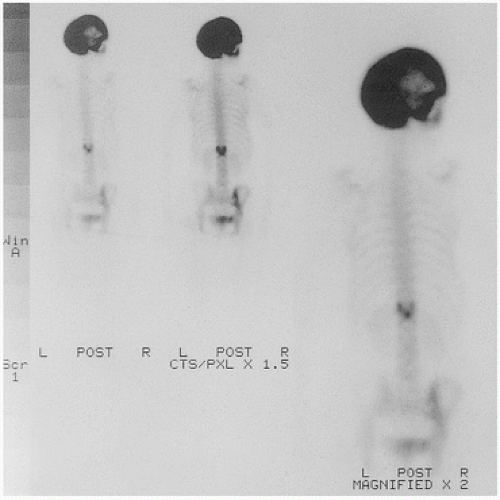
to fracture, and, in fact, fracture may be the presenting symptom. Other associated changes include calcific periarthritis and uremia, neurologic symptoms including deafness, vascular steal syndromes, platybasia (flattening of the base of the skull), and basilar invagination. Immobilization can lead to hypercalciuria, hypercalcemia, and nephrolithiasis. Roentgenographically, the changes are variable, depending on the stage and site of involvement (Fig. 4.16). Purely lytic lesions in Paget’s disease have been described, particularly in the skull, so-called osteitis circumscripta. In the initial stages of Paget’s disease in long bones, a lytic wedge advances along the cortex of the bone. Biopsies at this stage may show predominantly osteoclastic bone resorption mimicking hyperparathyroidism. Eventually, the resorptive phase progresses to one of intense bone remodeling, including osteoblastic bone formation. The resultant deterioration of the normal architecture of the bone leads to roentgenographically detectable coarsening of cortical bone, lack of demarcation between cortical and trabecular bone, and other such findings. The bone scan in this phase is intensely hot (Fig. 4.17). In many patients, Paget’s disease after a prolonged period of time enters a dormant phase, with little detectable osteoblast or osteoclast activity. In this stage, the disorder can be diagnosed by profound thickening and irregular cement lines throughout the involved bones. Fracture after biopsy of osteolytic Paget’s disease (82,83) and acute osteolysis after surgery potentially predisposing the patient to pathologic fracture (84) have been described.
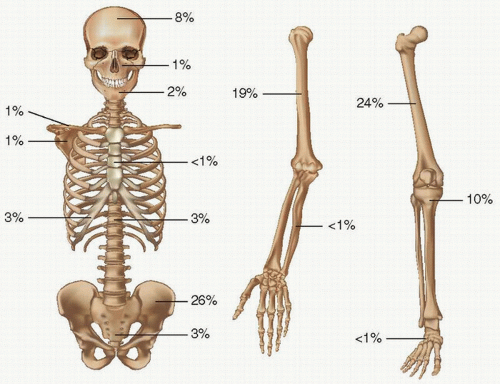
TABLE 4.6 Pagetic Involvement of Different Bonesa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
advancing flame or the tip of an arrow). The deformity in the contour of the bone is the consequence of fractures (complete or incomplete). Fractures in bones with Paget’s disease heal in an irregular fashion, resulting in deformities (osteitis deformans). The deformity in the contour of the end of the bone can at times result in nonunion, narrowing of the adjacent joint space, and osteophyte formation. This form of degenerative joint disease has been labeled Paget’s arthritis, and it can occur when only one bone is involved, even though it is more common when both bones surrounding a joint are affected.
formation and bone resorption. Bone trabeculae become both thick and thin. Cortical bone remodeling is also irregular, with loss of the distinction between cortical and trabecular bone. Osteoclasts are increased in number, large, and markedly multinucleated.
lines (Fig. 4.22) in now “burned-out” dense and (relatively) inactive tissue.
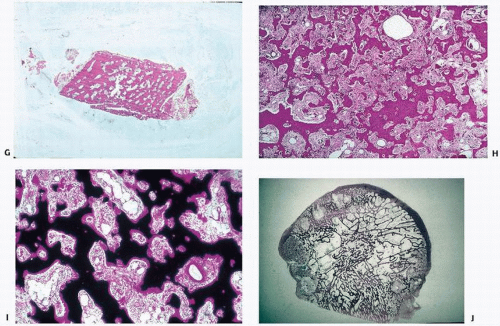 FIGURE 4.19. (Continued) • Poor cortical/cancellous bone demarcation (G) • Stromal fibrosis (H) • Increased vascularity (I) • Focal sharp demarcation from normal bone (J) |
TABLE 4.7 Iliac Trabecular Bone Morphology and Histomorphometry Data in Normal Bone and Case Examples of Idiopathic Osteoporosis, Osteomalacia, Secondary Hyperparathyroidism, and Paget’s Disease | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
inclusions have been shown in osteoclasts in Paget’s disease by Rebel et al. (87) and Reddy et al. (88). However, osteoclast inclusions are by no means specific and have been shown in giant cells in giant-cell tumors (89), pyknodysostosis (90), osteopetrosis (91), familial expansile osteolysis (92), and in macrophage-like cells in primary oxalosis (93).
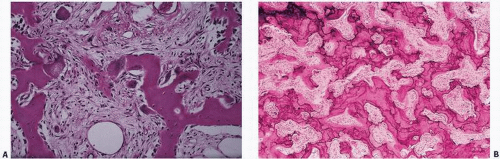 FIGURE 4.21. Active Paget’s disease with marked fibrosis and osteoclast activity (A) and pronounced irregular cement lines (B). |
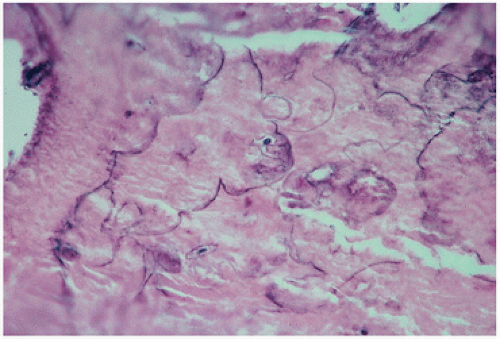 FIGURE 4.22. Inactive, “end-stage” or burned-out Paget’s disease. Only the curliform cement lines remain as a marker of the disease. |
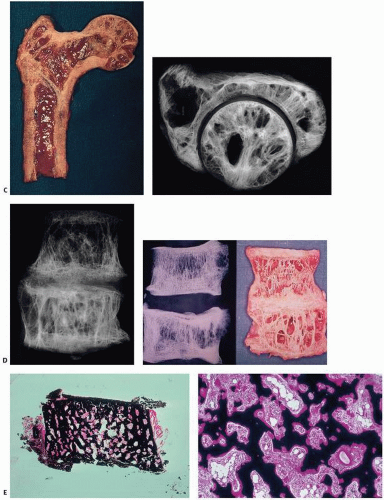
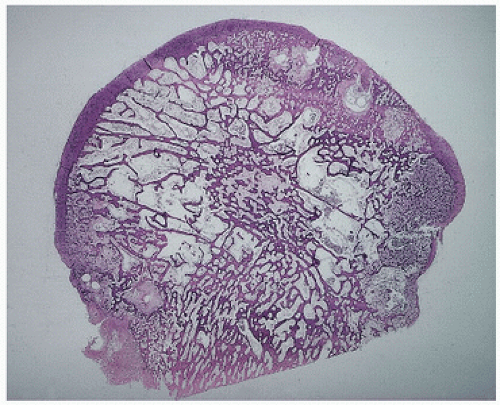 FIGURE 4.23. Coronal section through a femoral head with degenerative joint disease and Paget’s disease. The circumscribed dense areas represent pagetic foci. Intervening bone was nondiagnostic. |
Osteoclasts are irregularly shaped with multiple extensions and much infolding of cytoplasmic membrane. The infolding presumptively indicates abnormally increased surface activity and motility of these cells.
The presence of calcified fragments within the cytoplasm under the ruffled border shows that the cells may actually phagocytose whole pieces of bone, which is a highly abnormal form of bone resorption.
The presence of vesicular mitochondria suggests a high turnover rate for osteoclasts.
Nuclei are highly polymorphic and frequently contain many nucleoli. Nuclear inclusions are present in several nuclei per osteoclast and are filament-like structures. Some are microcylindric in shape. They are usually grouped together in parallel bundles and sometimes packed in paracrystalline arrays.
Cytoplasmic inclusions can be filament-like structures, some of which are organized into bundles, like those found in the nucleus. No paracrystalline arrays, however, are usually found in the cytoplasm. The cytoplasm may also contain envacuolated glycogen.
In the fibrous tissue surrounding the osteoclasts in Paget’s disease, cells similar to mononuclear osteoprogenitor cells are often found. These are often massed together and contain no nuclear inclusions.
TABLE 4.8 Paget’s Tumors | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
late stage at presentation compared with conventional osteosarcoma
higher percentage of metastatic disease at presentation
more rapid metastatic spread
higher risks in the use of chemotherapy due to higher age and poorer health of patients
risks associated with Paget’s disease-related high-out left ventricular heart failure
more axial location of Paget’s sarcoma compared with conventional osteosarcoma
harder detection of the tumors due to patient age-related mental frailty
desmoplastic fibroma (105). The most extensively evaluated benign lesion is the so-called Avellino tumor (Fig. 4.26).
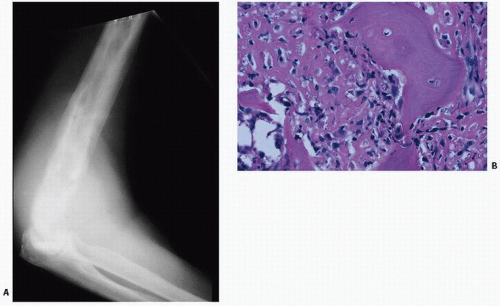 FIGURE 4.25. Paget’s sarcoma. Roentgenogram of Paget’s sarcoma of the humerus with superimposed destructive lytic lesion (A), proven to be an anaplastic sarcoma on biopsy (B). |
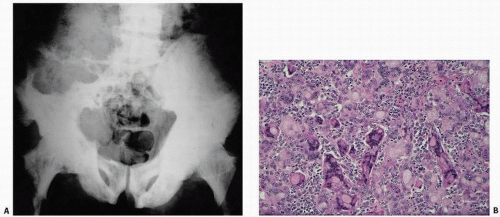 FIGURE 4.26. Giant-cell lesion in Paget’s disease. An 86-year-old white male immigrant from Avellino, Italy, with worsening pain in both extremities and weakness and progressive inability to ambulate. Physical examination revealed a large, left-sided, soft, immobile, painless mass predominantly involving the left buttock. Pelvic x-rays reveal diffuse pagetic changes of the pelvis, lumbar spine, sacrum, both pubic rami, and both proximal femora, and thickening of the iliopectineal line, all of which are classic for Paget’s disease of bone (A). A large lytic lesion was observed eroding the left iliac wing, L4, L5, and the sacrum. Computed tomogram was remarkable for a left-sided soft tissue mass invading the iliac crest, lumbar spine, and spinal canal. Pathology revealed multinucleated giant cells of various sizes and shapes, often with peculiar clear inclusions (B).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|