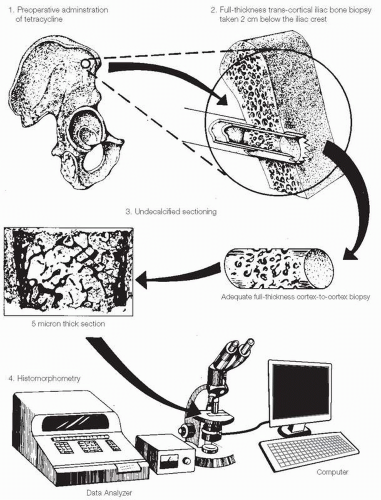
The diagnosis of osteoporosis is complicated by the fact that routine x-rays are not sensitive enough to pick up osteoporosis at an early age or stage and there are no laboratory tests that can help screen either for the diagnosis or for a population at risk. It has been estimated that roughly 40 percent of the skeletal mass must be lost before an x-ray can begin to show signs of osteopenia. Osteoporosis does not reveal any consistent metabolic abnormalities detected by standard laboratory tests and certainly none to predict a fracture at any given time in a specific patient.
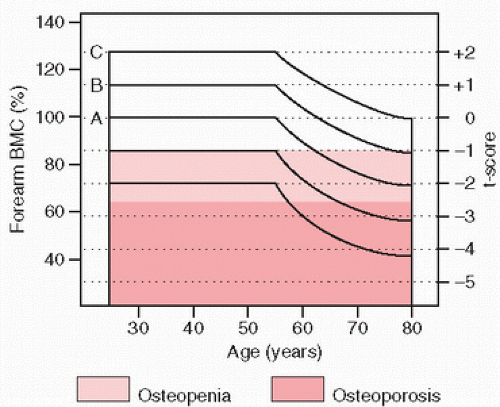
Bone loss appears to begin at about the age of 35 years, occurring at a rate of approximately 0.2 percent per year until menopause, at which time a precipitous decline ensues (
Fig. 3.8). Over time, there is an imbalance between bone formation and bone resorption, with bone loss increasing disproportionately. It has been estimated that over a lifetime, a woman loses approximately
35 percent of her cortical bone and 50 percent of trabecular bone. Therefore, bones with greater trabecular bone constituting their surface area, such as the vertebrae of the spine, may be preferentially affected (
Fig. 3.9). Whereas cortical bone predominates in the shaft of the long bone, trabecular bone predominates in the pelvis, flat bones, and ends of long bones. Being more metabolically active, trabecular bone is more subject to change in variations in mineral homeostasis. Although osteoporosis usually develops as a systemic loss of bone, it may occur regionally (
14) and at different rates in the cortical and trabecular bone compartments. In fact, two types of osteoporosis have been proposed on the basis of this compartmentalization of bone. For cortical bone loss, deterioration begins around age 40 years in both sexes at a rate of 0.4 percent per year, increasing with age until slowing or cessation later in life. In women, there is an accelerated superimposed cortical bone loss following menopause with the rate of loss approximately 2 to 3 percent per year, decreasing for over a decade. In addition, there is a loss of hematopoietic bone marrow throughout the skeleton, although it occurs at different rates in different anatomical sites. As bone marrow is increasingly exploited for its source of mesenchymal stem cells (and osteoblast precursors), we have learned that age- and site-specific stem cell depletion may be a significant contributory risk factor.
To summarize, bone loss occurs throughout aging, beginning approximately at age 35 years, and affects both cortical and trabecular bone. There are different rates of loss in different parts of the skeleton. In women, the superimposed accelerated loss at or about menopause leads to considerable bone loss over a period of 10 years, producing the now well-recognized significant risk for fractures and their associated morbidity and mortality.
Genetics
Estrogen deficiency and genes involved in sex steroid metabolism such as those coding for aromatase (CYP19 genes) and other sex steroid hormone receptors are probable contributory factors in the expression of osteoporosis (
15). For example, the CAG repeat polymorphism (CAGR) in the androgen receptor gene has been associated with decreased BMD and the increased incidence of vertebral fractures in males.
Additional osteoporosis candidate genes are being investigated (
16). X-linked traits with osteoporosis and fractures have been reported for over 35 years and most recently in five families with pathogenic variants of plastin 3 (PLS3), a protein involved in the formation of filamentous actin (F-actin) bundles, which are important in bone health (
17).
Vitamin D receptor gene polymorphisms have been shown to affect BMD variation and fracture rates, and may interact with calcium and estrogen to modulate BMD. Studies of collagen genetics suggest that an Sp1 polymorphism in the COL 1A1 gene may be associated with osteoporotic fractures—not surprising, because collagen 1 defects are ubiquitous in osteogenesis imperfecta. Because high serum homocysteine levels have adverse effects on bone methylene tetrahydrofolate reductase (MTHFR). which affects the methylation of homocysteine to methionine, they may be an important contributor to the development of osteoporosis. Other candidate factors include ApoE, a lipid-transporting glycoprotein; insulin-like growth factor 1 (IGF-1), a growth factor related to growth plate chondrocyte proliferation and matrix mineralization; interleukin 6 (IL-6), a cytokine that promotes osteoclast differentiation; LRP5, whose mutations can lead to both high- and low bone mass syndromes; and SOST, a gene that can lead to sclerosteosis, a bone dysplasia characterized by bone overgrowth.
Most clinical and experimental research has historically focused around the contributions of calcium or estrogen deficiency in causing osteoporosis (
18) and the disturbance of the bone remodeling cycle. Although there is no definitive or widely accepted evidence to suggest that calcium deficiency is directly related to the production of osteoporosis, there exists a substantial body of scientific literature supporting an important role for calcium. Classic studies of bone status and fracture rates from an epidemiologic point of view are convincing. In a classic study in the former Republic of Yugoslavia, a lower fracture rate was noted in patients coming from areas of dietary high-calcium intake compared with the fracture rates in low-calcium dietary regions (
19).
There is little question that the development of osteoporosis in women is temporarily linked to the postmenopausal state, a period of estrogen deficiency due to either waning ovarian function or amenorrhea. We now can construct a larger number of risk factors based on the clinical profiles of women with osteoporosis. The typical osteoporotic patient is usually a white or Asian postmenopausal woman of lean body build with a positive family history, low dietary calcium intake, and a history of relatively little weight-bearing physical activity. Significant contributing factors include hyperparathyroidism or increased thyroid activity, including thyroid medication as well as corticosteroids or anticonvulsive therapy.
Parathyroid hormone may have contrasting effects. Whereas continuous elevated parathyroid secretion as seen in parathyroid
adenomas is linked to increased bone resorption, transient or intermittent administration of parathormone (PTH) can have a stimulatory effect on bone formation, the latter providing a scientific basis for new treatments of osteoporosis with PTH (
20).
Perhaps the most important risk factor is the history of a previous fragility fracture. Fractures do predict fractures in the aging and postmenopausal states. The risk of future fractures after having sustained an osteoporotic fracture increases from 1.5- to 9.5-fold (
21).
Other risk factors for osteoporosis include smoking, caffeine intake, alcoholic intake, and high-protein diets.
Cigarette smoking decreases BMD and increases the risk of nonunion and delayed union of fractures. Nicotine and carbon monoxide decrease microperfusion and tissue oxygenation, increase platelet aggregation and blood viscosity, resulting in microclotting (
22) and, therefore, potential osteonecrosis. Soft tissue and wound healing is also impaired, and smokers are at greater risk for infection, back pain and degenerative disc disease, rheumatoid arthritis, and even rotator cuff tears.
Gastrectomy may also adversely affect the bone mass. Bauer et al. (
23) have estimated risk factors, in decreasing strength of positive association, estrogen use, non-insulin-dependent diabetes, thiazide use, increased weight, greater muscle strength, later age at menopause, and greater height. The effects of growth hormone and acromegaly are complex and less clear. Androgens, such as testosterone, lead to increased bone mass (
22).
Other risk factors include bone ischemia as typically seen in conditions such as regional or transient osteoporosis, hip axis length, and skeletal age at maturity. Associations with hemosiderosis, fatty marrow, arthritis, Marfan syndrome, depression, and pregnancy have been made. Increased body weight, exercise, or obesity may be protective. With disuse and the skeletal deloading effects of microgravity, osteoporosis occurs. For a long time, NASA scientists worried that astronauts, losing 1 to 2 percent of their bone density per month in space, would return to earth with weaker bones. Improved exercises on treadmills in space and antiosteoporotic drugs have lessened that concern. Increased bone mass in the dominant arm in sports such as tennis, squash, and baseball is well documented. Whether diabetes is associated with bone loss or gain is still debatable.
The effect of glucocorticoids has been most extensively studied (
24). Both a decrease in bone formation and an increase in bone resorption have been postulated. A downregulation of the synthesis of extracellular matrix proteins and integrins, cell-surface receptors, by osteoblasts has been demonstrated experimentally (
25). In addition, tissue culture experiments have demonstrated glucocorticoid-stimulated increases in osteoclasts and measurable release of calcium into culture medium.
Dexamethasone has been found to upregulate the expression of the osteoblast inhibitory factor Dickkopf 1 (or DKK-1), which interacts with the Wnt coreceptors LRP5 and LRP6, thereby inhibiting the Wnt signal (
24). In addition, glucocorticoids suppress the expression of RUNX2/Cbfa1 critical for osteoblastogenesis. Other actions include suppression of insulin-like growth factor and osteoclastic promotion via increasing the expression of RANKL and inhibiting the expression of osteoprotegerin, the osteoclast decoy signal.
A typical osteoporotic profile is that of a woman of short stature with low body weight and low-calcium dietary intake, who is a heavy smoker. Norwegians have estimated that lean women who smoke have essentially added 10 years to their “bone age” (
26). Daughters of women with osteoporosis have reduced bone mass in the lumbar spine and perhaps the femoral necks, suggesting, at least in some studies, a genetic factor to the transmitted risk for osteoporosis.
Postmenopausal osteoporosis may also result from a relatively low peaked bone mass at skeletal maturation, a factor that is often implicated in the lack of significant osteoporosis in the average black woman. Blacks, especially black women, have less osteoporotic fractures at comparable ages than whites.
Of particular interest is the association of lactase deficiency with patients with the spinal fracture compression syndrome and non-ankle weight-bearing bones (
27). It is reasonable to presume that the avoidance of milk products associated with this condition could lead to low bone mass as a result of long-term suboptimal calcium ingestion in the diet. The association of lactose deficiency with osteoporosis is controversial in that populations where the prevalence of lactase deficiency is generally highest are those in black Americans and sub-Saharan Africa.
Mechanical loading on the skeleton is important in maintaining bone density and strength. Well-known clinical observations include the precipitous loss of bone in astronauts in space and the rarity of osteoporosis in the obese. In fact, bone loss during diet-induced weight loss in the obese has been considered a physiologic normalization. Lack of exercise is a significant factor in osteoporosis observed in men and women.
Mechanical loading is thought to have a protective effect by maintaining bone remodeling in its normal coupling phenomenon: In non-weight-bearing bones, bone resorption is increased and bone formation is decreased. Direct stimulation of osteoblasts in mechanical loading has been postulated.
The osteoporotic man, a rarer phenomenon, is more difficult to characterize although glucocorticosteroids, whether endogenously produced or therapeutically given, alcohol intake, hypogonadism, inactive lifestyle, osteomalacia, and neoplasia are important factors (
15). Greater bone mass at all ages, shorter life expectancy, and no equivalent to menopause have been used as explanations that osteoporosis is less common in men. Although hip fracture rates are indeed higher in women than in men, with increasing age, the lifetime risk of fractures of the femur is substantial in the male population. The major factors for hip fracture appear to be heavy alcoholic use. In a well-studied medical chart review in a veteran population, preadmission ambulatory problems, confusion, and overall low body mass have been documented. These studies confirm known traditional risk factors for the osteoporotic hip fractures in women, which include medical conditions predisposing to falls such as neurotropic cerebrovascular and cardiovascular arrhythmias, environmental hazards leading to tripping and falls, and medications that induce decreased alertness, dizziness, and impaired vision.
Osteoporosis in the pediatric age group remains the most perplexing group of all, but certainly includes subtle cases of osteogenesis imperfecta.
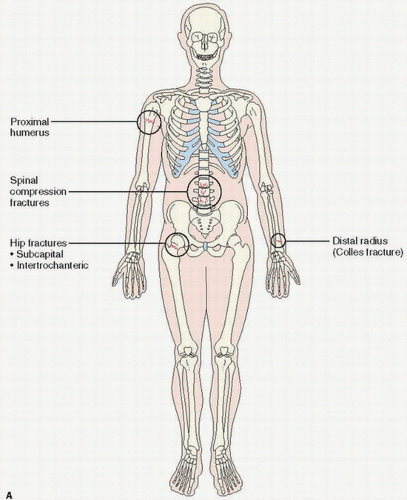
From a clinical point of view, osteoporosis is suspected in any elderly patient with a hip fracture and any postmenopausal woman complaining of back pain or recent or gradual loss of height. Although the causes of back pain are protean, osteoporosis should be suspected in a woman with progressive thoracic kyphosis—a dowager’s hump—especially if she has the risk criteria mentioned before. The occurrence of low trauma fracture almost anywhere in the skeleton is associated with future substantial risk of fracture. Healy and Lane (
28) have emphasized scoliosis as an important complication in these women. As mentioned, the concern about
osteoporosis is directly linked to its relationship to the development of skeletal fractures. In one large prospective cohort study, it was shown that with age, in non-black women older than 65 years of age, the risk of fractures of the wrist, foot, humerus, hip, toe, leg, pelvis, hand, and clavicle was significantly related to reduced bone mass. Fractures of the ankle, elbow, finger, and face, however, were not significantly at risk for loss of bone mass. In this regard, an enormous effort has been undertaken to perfect early roentgenographic diagnosis.
Radiographs and Imaging Procedures
Routine x-rays are not sensitive enough to diagnose osteoporosis before substantial bone loss has occurred (
Fig. 3.9). Nonetheless, analysis and semiquantitative measurements of roentgenograms of the femur, vertebra, calcaneus (
29), and hand have been proposed as well as ultrasound analyses (
30). The most popular measurement today is that of bone densitometry (vida infra).
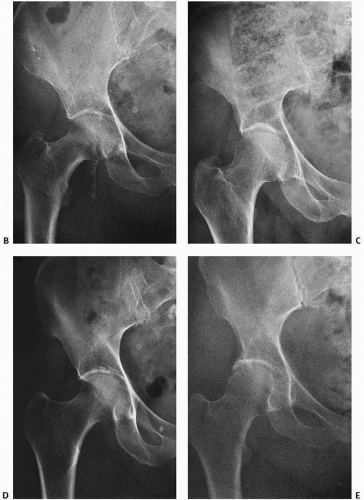
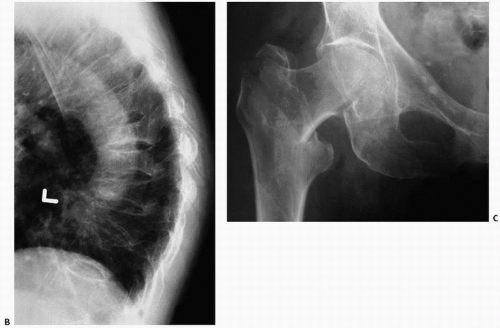
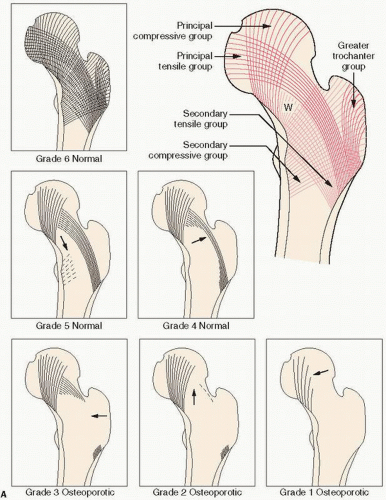
Of historical interest is the Singh index, a measurement of trabecular bone patterns of the proximal femur (
31) (
Fig. 3.11). In this technique, one evaluates the internal architecture of the proximal femur, which is composed of two major trabecular systems produced during weight bearing. The Singh index proposes that, with increasing osteoporosis, bone loss follows a predictable pattern with loss of secondary trabeculae first. Ward triangle enlarges, and then there follows loss of tensile trabeculae, and finally compressive trabeculae. The preferential pattern of loss is thought to reflect the mechanical means of weight bearing. A comparable system has been used in the calcaneus.
Meema and Meindok (
32) have extensively evaluated peripheral skeletal measurements using an x-ray of midshaft of the index finger metacarpal measuring the total diaphyseal width and combined cortical thickness, with fractions of the latter over the former being used as an osteopenia index (
Fig. 3.12).
In general, there are three methods (
Table 3.7) for the noninvasive quantitative estimation of bone loss: (a) routine imaging procedures, which are descriptive in nature and used to evaluate items such as the presence or absence of bone trabeculae, or cortical bone thickness; (b) semiquantitative procedures, which use routine x-rays to grade trabecular bone patterns, such as the Singh index or measurement of the thickness of cortices; and (c) quantitative procedures, which are utilized to attempt to measure bone density at different sites with techniques using ultrasound or various types of radiation. Of these, DEXA is considered the current gold standard (
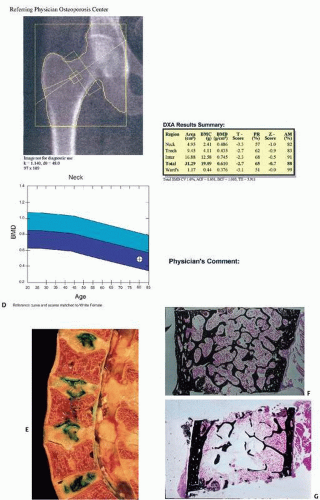
Fig. 3.13).
In DEXA, x-ray beams of two distinct energies are passed through a specific region of bone, and differences in the absorption of each beam are computer analyzed to distinguish between the mineral content of bone and that of adjacent tissues to determine bone density. Although most DEXA is used to measure BMD of the lumbar spine and hip, it has been shown to correlate with heel ultrasound in discriminating a first hip fracture (
30).
The frequency with which DEXA testing should be performed is currently controversial. In 2012, Medicare paid for bone density testing every 2 years. Many medical groups recommend testing as early as 50 years of age, but there are little data to guide such decisions. It has been estimated that osteoporosis would develop in less than 10 percent of older, postmenopausal women during rescreening intervals of approximately 15 years for women with normal bone density or mild osteopenia, 5 years for women with moderate osteopenia, and 1 year for women with advanced osteopenia (
33).
Limitations in the DEXA measurement of bone are well known. DEXA provides only a two-dimensional assessment of bone, a three-dimensional tissue. Disturbances in three-dimensional geometry and microarchitectural deterioration are not captured. DEXA scans can also be distorted by tissue calcifications such as those seen in aortic calcification.
Specific examples of DEXA’s limitations are seen in
Figure 3.4A, in which DEXA would measure a normal amount of bone but the tissue is clearly osteomalacic. DEXA would also miss the profound trabecular bone osteoporosis in
Figure 3.7G because there is a significant amount of cortical bone.
Thus, BMD as measured by a technique such as DEXA is a limited predictor of fracture risk. To improve the ability or predict fracture risk, information in addition to BMD is needed such as analyses of microarchitectural structure and material properties of the bone.
Both ultrasound (
30) and DEXA have been applied to other bone sites such as the calcaneus in an attempt to quantify bone mass and predict fracture risk. In general, the specificity of calcaneus DEXA measurements to predict osteoporosis at the axial sites such as the hip or spine is excellent but sensitivity poor.
With specific regard to the foot, foot fractures are more likely to be osteoporotic in etiology than ankle fractures (
34).
When taking into consideration increasing numbers of known risk factors for osteoporosis, low BMD as assessed by DEXA has been correlated with a higher rate of hip fractures (
35).
In quantitative computed tomography (CT), the attenuation of an x-ray beam as it passes through a bone usually L1 to L4 is used. It is related to bone density using a phantom that includes a bone mineral, K2HPL4, and soft tissue, water, and glycerol equivalent. Despite widespread use of these techniques for investigational purposes, the search for a fracture threshold has been elusive. This is readily explained by the complex etiology of fracture in which bone mass is only one contributing factor. Other important considerations are the degree of trauma, the mechanical strength of the remaining bone, the overall structural integrity of the skeleton in other locations, and the aforementioned contribution of lifestyle risk factors. Nevertheless, values do exist in these diagnostic modalities at which severe osteoporosis may be presumed to be present (
Table 3.8) (
36). Caution is advised in extrapolating bone mass measurement at one site to be representative of bone mass at other sites.