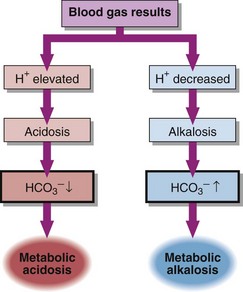
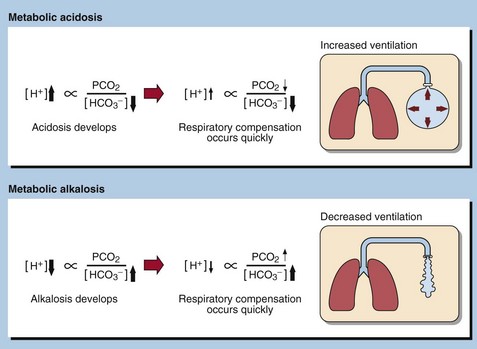
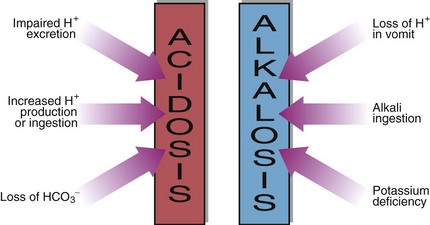
21 Metabolic acid–base disorders are caused by an increase in H+ production or a loss of H+ triggering compensatory mechanisms that result in the loss or gain of HCO3−. Direct loss or gain of HCO3− will also cause metabolic acid-base disorders. Primary metabolic acid–base disorders are recognized by inspecting the bicarbonate concentration (Fig 21.1). Respiratory compensation takes place quickly so patients with metabolic acid–base disorders will usually show some change in blood PCO2 because of hyperventilation or hypoventilation (Fig 21.2). In a metabolic acidosis the primary problem is a reduction in the bicarbonate concentration of the extracellular fluid. The main causes of a metabolic acidosis are shown in Figure 21.3. These are:
Metabolic acid–base disorders
Metabolic acidosis
 increased production of hydrogen ions
increased production of hydrogen ions
 ingestion of hydrogen ions, or of drugs that are metabolized to acids
ingestion of hydrogen ions, or of drugs that are metabolized to acids
 impaired excretion of hydrogen ions by the kidneys
impaired excretion of hydrogen ions by the kidneys
 loss of bicarbonate from the gastrointestinal tract or in the urine.
loss of bicarbonate from the gastrointestinal tract or in the urine.
Metabolic acid–base disorders