45 Menstrual cycle disorders
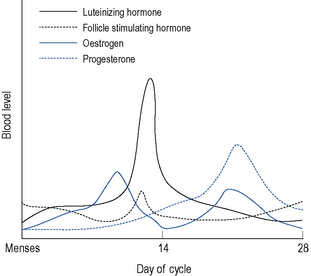
Menstruation itself occurs as a result of cyclic hormonal variations (Fig. 45.1). During the first half or follicular phase of the menstrual cycle, the endometrium thickens under the influence of increasing levels of oestrogen (most notably estradiol, which at the peak of its preovulatory surge reaches around 2000 pmol/L) secreted from the developing ovarian follicles. Once the serum oestrogen level has surpassed a critical point it triggers, by positive feedback, the anterior pituitary to release, about 24 h later, a surge of luteinising hormone (LH; up to 50 iu/L) and after 30–36 h, ovulation follows.
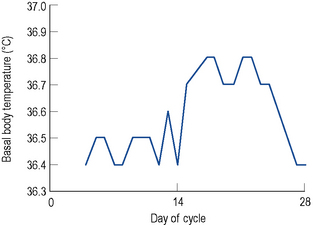
After ovulation, which occurs around day 14 of a 28-day menstrual cycle, and as the luteal phase progresses, the endometrium begins to respond to increasing levels of progesterone. Both progesterone and oestrogen are secreted from the corpus luteum which is formed from the remains of the ovarian follicle after ovulation. The lifespan of the corpus luteum is remarkably constant and lasts between 12 and 14 days; hence, the length of the second half or the luteal phase of the menstrual cycle is between 12 and 14 days. Between days 18 and 22 of a 28-day cycle, both sex steroids peak, with levels of progesterone reaching around 30 nmol/L. As progesterone has a thermogenic effect upon the hypothalamus, basal body temperature increases by about 1 °C in the second half of an ovulatory cycle (Fig. 45.2). Most ovulatory cycles range from 21 to 34 days.
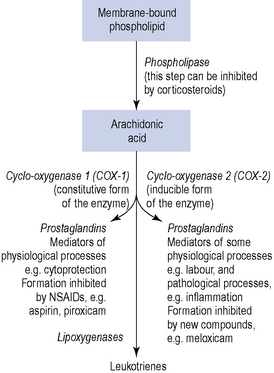
There is evidence which suggests a physiological and pathological role for the local hormones, known as prostaglandins, in the process of menstruation. Prostaglandins are 20-carbon oxygenated, polyunsaturated bioactive lipids, which are cyclo-oxygenase-derived products of arachidonic acid. Indeed, both the myometrium and the endometrium are capable of synthesising and responding to prostaglandins. A potential role for another family of autocoids, the leukotrienes, in the regulation of uterine function remains uncertain, although it is known that leukotrienes can also be synthesised from arachidonic acid by lipoxygenase enzymes (Fig. 45.3).
Premenstrual syndrome (PMS)
PMS encompasses both mood changes and physical symptoms. Symptoms may start up to 14 days before menstruation, although more usually they begin just a few days before and disappear at the onset of, or shortly after, menstruation. However, for some women, the beginning of menstruation may not signal the complete resolution of symptoms. Numerous studies have demonstrated that this condition can cause substantial impairment of normal daily activity, including reduced occupational activity and significant levels of work absenteeism. Severity varies from cycle to cycle and may be influenced by other life factors such as stress and tiredness. The most severe form of PMS may be referred to as premenstrual dysphoric disorder (PMDD) as defined by the Modified Diagnostic and Statistical Manual of Mental Disorders appendix IV, or DSM-IV (American Psychiatric Association, 2000) and for which the criteria are set out in Box 45.1. Other bodies (American College of Obstetricians and Gynecologists, 2000) have published diagnostic criteria for PMS (Box 45.2). There is considerable overlap between PMS and PMDD.
Box 45.1 Summary of DSM-IV diagnostic criteria for premenstrual dysphoric disorder (PMDD)
Seriously interferes with work, social activities, relationships
Not an exacerbation of another disorder
Confirmed by prospective daily ratings during at least two consecutive symptomatic cycles
Box 45.2 Diagnostic criteria for premenstrual syndrome (PMS)
| Affective | Somatic |
|---|---|
| Depression | Breast tenderness |
| Angry outbursts | Abdominal bloating |
| Irritability | Headache |
| Anxiety | Swelling of extremities |
| Confusion | |
| Social withdrawal |
Symptoms relieved within 4 days of menses onset without recurrence until at least cycle day 13
Symptoms occur reproducibly during two cycles of prospective recording
Patient suffers from identifiable dysfunction in social or economic performance
Aetiology
Vitamins and minerals
Pyridoxine phosphate is a co-factor in a number of enzyme reactions, particularly those leading to production of dopamine and serotonin (5-hydroxytryptamine). It has been suggested that disturbances of the oestrogen/progesterone balance could cause a relative deficiency of pyridoxine, and supplementation with this vitamin appears to ease the depression sometimes associated with the oral contraceptive pill. Decreased dopamine levels would tend to increase serum prolactin, and decreased serotonin levels could be a factor in emotional disturbances, particularly depression. There is some evidence that premenstrual mood changes are linked to cycle-related alterations in serotonergic activity within the CNS and, therefore, serotonin may be important in the pathogenesis of PMS. There are also data to suggest that a variety of nutrients may play a role in the aetiology of PMS, specifically calcium and vitamin D. Further hydroxylation of 25-hydroxyvitamin D3 (25(OH)D3) takes place in target tissues which include the breast and the endometrium. In the Nurses’ Health Study II (NHSII), high total vitamin D intake lowered risk of PMS by almost a third (Bertone-Johnson et al., 2005). Oestrogen influences calcium metabolism by affecting intestinal absorption, and parathyroid gene expression and secretion, so triggering fluctuations throughout the menstrual cycle. Disruption of calcium homeostasis has been associated with affective disorders.
Symptoms
Symptoms occur 1–14 days before menstruation begins and disappear at the onset or shortly after menstruation begins. For the rest of the cycle, the woman feels well. Symptoms are cyclical, although they may not be experienced every cycle, and can be either physical and/or psychological (see Boxes 45.1. and 45.2 for symptomatology). The lives of the 5% or so of women who are severely affected may be completely disrupted in the second half of the menstrual cycle. The symptoms of PMS tend to decrease as a woman gets closer to the menopause as her ovulatory cycles become less frequent.
Management
Pharmacological management
Combined oral contraceptives (COC)
It is thought that use of third-generation progestogens is associated with increased resistance to the anticoagulant action of activated protein C. Oral contraceptive treatment diminishes the efficacy with which activated protein C down regulates in vitro thrombin formation. This is known as activated protein C resistance and is more pronounced in women using the COC pills containing desogestrel than in women using those containing levonorgestrel. However, it has also been recognised that women who do react to third-generation progestogens with venous thromboembolism may be revealing a latent thrombophilia. There are several conditions, congenital or acquired, that can cause thrombophilic alterations. A genetic factor known as factor V Leiden mutation is the most common inherited cause of thrombophilia, and this mutation results in resistance to the effects of activated protein C. Carriers of this mutation have more than a 30-fold increase in risk of thrombotic complications during oral contraceptive use, although this has been disputed (Farmer et al., 2000) because no increase in risk of venous thromboembolism was found with the third-generation progestogens. In conclusion, if there is a history of thromboembolic disease at a young age in the immediate family, then disturbances of the coagulation system must be ruled out.