and Victor G. Prieto2
(1)
Division of Dermatopathology, Miraca Life Sciences, Dallas, TX, USA
(2)
MD Anderson Cancer Center, University of Texas, Houston, TX, USA
Introduction
Melanoma is a relatively common malignant neoplasm that arises from melanocytes and is most commonly cutaneous in origin; however, it can arise in the eye, mucosae, and internal organs [1, 2]. Melanoma is one of the most common forms of cancer in young adults and thus it represents a major public health problem. Overall, melanoma is the fifth most common cancer in men and the sixth in women in the United States. The incidence of cutaneous melanoma has increased dramatically in the last years and remains to be the leading cause of death in cutaneous malignancies [3–6]. The Surveillance, Epidemiology, and End Results (SEER) Program reports an increase of more than 600 % in the diagnosis of cutaneous melanoma from 1950s to 2000s [7], with an average increase of 2.9 % each year between 1985 and 2009. Overall, the lifetime risk for developing melanoma in patients born in 2012 is 2.62 for men and 1.63 for women (SEER data) . The increased incidence varies by age, gender, ethnicity, and histologic subtype. Before the age of 40, the incidence is higher in women, with most melanomas located on the lower extremities and exhibiting the superficial spreading histology. After the age of 40, the incidence is much higher in men, with most melanomas presenting on the head and neck and back. Also, incidence increases are most pronounced in white populations, particularly elderly individuals, whereas the incidence among darkly pigmented populations has risen only slightly or remained stable. The incidence of melanoma in children is increasing [8–10], which can be associated with risk factor such as xeroderma pigmentosum, familial dysplastic nevus syndrome or familial melanoma, and possibly immunosuppression.
Interestingly, there appears to be discordance between incidence and mortality trends in melanoma. While mortality rates increased slightly in the United States and Europe during the 1970s and 1980s, a stabilization of mortality rates was observed in most countries during the 1990s and 2000s. This difference in incidence and mortality trends may be due to the fact that more patients present at an earlier stage with smaller lesions that are potentially curable although it is also possible that changes in histologic criteria may have resulted in overdiagnosis of melanoma [11–16]. While these factors likely play some role, they certainly cannot explain entirely the observed incidence rise in patients presenting with advanced tumors suggesting a true increase in melanoma incidence [17, 18].
Risk Factors
Understanding the risk factors for the development of melanoma is important. Individual risk assessment influences clinical decision-making including the threshold for performing a biopsy, prevention counseling, and surveillance. The etiology of melanoma is multifactorial including demographic, environmental, and genetic risks. However, the majority of melanomas are likely related to ultraviolet radiation, since UV-related mutations are relatively common in melanoma [19].
Demographic Risk Factors : Age is a well-known risk factor for the development of melanoma as its incidence increases with age [20]. Although the median age of melanoma patients is about 50 years, it occurs in a wide age distribution [21]. The incidence is greatest in older patients, but it is also one of the most common cancers in young adults and adolescents. When compared to other cancers, due to the relatively young median age of melanoma patients, melanoma ranks among the worst in terms of lost productive years. The risk for melanoma is greatest in ethnic groups with lighter skin types, with the majority of melanomas in the United States diagnosed in non-Hispanic, white patients. On the other hand, in the United States, African Americans and Hispanics present with higher-stage melanomas and have a worse 5-year survival compared to white populations. The lifetime risk for melanoma is higher in men than in women, but it is also affected by sex. Before age 40, incidence rates are higher in women than in men; after age 40 years, rates are almost twice as high in men as in women.
A personal history of melanoma confers an increased risk for developing an additional melanoma. Patients with a prior melanoma have an approximately 2–5 % chance of developing a subsequent melanoma. Regarding histologic subtypes, patients whose first melanoma is lentigo maligna melanoma or nodular melanoma have a higher risk of a subsequent primary melanoma, compared with superficial spreading melanoma. A personal history of squamous cell carcinoma or basal cell carcinoma triples or doubles (respectively) the risk of developing melanoma. The risk is slightly lower in patients with actinic keratosis.
Environmental Risk Factors : It is well accepted that exposure to ultraviolet (UV) radiation is a major risk factor, especially among those with fair skin who are more susceptible to sunburns. As such, individuals who are most susceptible to the effects of excessive sunlight (e.g., blond or red haired, blue eyed, or with fair complexions) that tan poorly and tend toward sunburn have an increased risk of melanoma [22, 23]. While UVB has been regarded as the causative agent, UVA radiation is also carcinogenic and can induce melanoma [24, 25]. There is an increased incidence of melanoma in people that live close to the equator or at high altitudes and, in general, in persons who report increased exposure to UV radiation. The risk from UV radiation varies according to intensity, frequency, and age at the time of exposure. Intermittent and intense UV radiation exposure and sunburns are risk factors. Studies have shown UV radiation exposure during childhood is particularly associated with increased risk; however, sunburns occurring during any period of life also increase the risk of melanoma. The most common anatomic location of melanoma for men is the back and in women the legs , finding that has been suggested to be due to intermittent and intense UV radiation in each sex. Exposure to artificial UV radiation also increases melanoma risk, particularly tanning salons [26].
Presence of Nevi and/or Dysplastic Nevi : Large numbers of standard nevi, of large size, and presence of dysplastic nevi are risk factors for melanoma [27]. The presence of >10 clinically dysplastic nevi confers a 12-fold increased risk. Also increased numbers of conventional nevi and nevi greater than 6 mm in diameter are independent risk factors for the development of melanoma [27–30]. However, although the presence of non-dysplastic and dysplastic nevi predicts an increased risk for melanoma, only a small percentage of nevi progress to melanoma; approximately 25 % of melanomas arise in association with a melanocytic nevus [31]. Some authors have observed that younger age, superficial spreading histologic subtype, and trunk location are predictors of a melanoma being histologically associated with a melanocytic nevus [31].
Family History : A prior family history of melanoma increases the risk for acquiring melanoma. The risk of developing melanoma is 2.62 times higher when a parent has had melanoma and is 2.94 times higher if the melanoma was in a sibling. Overall, about 8 % of people newly diagnosed with melanoma have a first-degree relative with melanoma, and about 1–2 % has two or more close relatives with melanoma. Fewer than 10 % of patients present with true familial or hereditary melanomas in which there is a strong family history or a documented melanoma-related, germline mutation. These families have autosomal dominant inherited susceptibility mutations and develop single or multiple melanomas at early age [32].
Several genetic loci determine susceptibility to melanoma, with the most important of these being CDK4 and p16/CDKN2A located on chromosome 9p21, both being high-risk genes [33]. Both CDNKN2A and CDK4 are highly penetrant, susceptibility genes and result in the majority of familial melanomas. The CDKN2A gene encodes two proteins, p16 and p14 ARF, which are cell cycle inhibitors and are potent tumor suppressors. The CDKN2A mutation is present in up to 40 % of families with three or more cases of melanoma, whereas CDK4 mutations are present in far fewer families. The risk of developing melanoma in a patient who is a CDKN2A mutation carrier is between 30 % by age of 5 years and 67 % by age of 80 years. This risk varies by geographic location due to environmental factors, such as UV radiation, since the latter appears to play a significant role in susceptible families [34]. In addition, the same risk factors that influence the incidence of melanoma (such as increased number of nevi, sunburn, etc.) also increase the penetrance in CDKN2A mutation carriers. Melanoma-prone families with CDKN2A germline mutations have an increased risk of developing other neoplasms, primarily pancreatic cancer, with variable penetrance [33]. Germline CDK4 mutations in familial melanoma account for approximately 1 % of all familial melanomas [33]. While less frequent than CDKN2A mutations, CDK4 mutations carry an equally high risk for the development of melanoma and show a similar clinical phenotype [35]. In contrast to CDKN2A and CDK4, heritable mutations in the melanocortin-1 receptor (MC1R) gene have low penetrance [36]. MC1R mutation is commonly associated with a red hair phenotype, but it confers an increased risk of developing melanoma even in non-red-haired individuals. The risk of developing cutaneous melanoma lies between 1.5-fold and 3-fold for patients with MC1R variants, but when both alleles are mutated, it increases 17-fold for development of BRAF mutant melanomas. Some familial melanomas occur in the setting of dysplastic nevus syndrome. A family history of melanoma in multiple first-degree relatives and younger age at diagnosis are important features of this syndrome. Families who present with the dysplastic nevus syndrome may also have CDKN2A mutations. Other heritable disorders that predispose patients to melanoma include xeroderma pigmentosum, Li-Fraumeni syndrome, BRCA2, or familial retinoblastoma.
Histologic Parameters Reported in Melanoma
Accurate and thorough histopathological diagnosis of melanocytic lesions is essential for the clinical management of the patients, since the reporting of these histopathologic parameters, especially in the initial biopsies, is a critical component of both diagnosis and staging [37]. Institutions and dermatopathologists have different styles and variables in regard to the routine reports of histologic parameters in invasive melanomas. Some of these reports have only minimal information (such as Breslow thickness), while others reports are quite comprehensive. The information in those detailed reports might not be of immediate relevance, but its importance might become apparent later, when analyzed in prospective or retrospective studies. As an example of this, mitotic activity count is now included as a histopathologic element in the AJCC melanoma staging and classification system [37]. In our practices, we provide a report that includes all the histologic parameters that have been proved to be significant for staging and prognosis, as well as additional histologic features that may potentially be helpful (see Table 7.1).
Table 7.1
Synoptic report of melanoma
Malignant melanoma, invasive, type |
Clark level |
Breslow thickness, mm |
Radial (non-tumorigenic) growth phase |
Vertical (tumorigenic) growth phase |
Mitotic figures/mm2 |
Ulceration |
Regression |
Vascular invasion |
Perineural invasion |
Microscopic satellitosis |
Tumor-infiltrating lymphocytes |
Associated melanocytic nevus |
Predominant cytology |
Surgical margins |
Cutaneous melanomas are classified into four histologic subtypes : superficial spreading, lentigo maligna, acral lentiginous, and nodular type. While technically speaking the histologic subtype classification does not seem to affect prognosis, its proper classification is important for other reasons. One benefit of histologic classification is to provide subtyping for epidemiologic studies. Also, there are emerging genetic studies that show distinctive patterns of chromosomal aberrations in melanomas arising on chronically sun-exposed skin versus melanomas arising in areas with intermittent sun exposure or acral/mucosal areas. These heterogeneous molecular changes in melanoma are of clinical relevance because they are likely to result in different targeted therapies; acral lentiginous and mucosal melanomas can harbor KIT gene mutations and can potentially respond to targeted therapies with tyrosine kinase inhibitors [38–40]. It is also important to recognize that desmoplastic melanomas have higher propensity for neurotropism with high local recurrence and that in cases of pure desmoplastic melanoma, there is a lower incidence of lymph node metastasis and with an outcome different from conventional melanomas [41].
Breslow Thickness
Numerous prognostic models have been developed in an attempt to predict which patients ultimately will develop advanced disease [42–45]. Identification of patients at high risk for advanced melanoma can help physicians plan appropriate surgery and possible adjuvant therapy. Virtually all studies conducted on primary cutaneous melanomas that have analyzed prognostic factors have shown that the most significant prognostic variable is Breslow thickness [46], which measures the vertical thickness of melanoma. The revised American Joint Committee on Cancer (AJCC) staging criteria accurately predict sentinel lymph node positivity in clinically node-negative melanoma patients. When grouped by AJCC cutoff points, there was an increased incidence of positive sentinel lymph nodes with increasing tumor thickness: 4 % in melanomas smaller than 1.00 mm, 12 % in melanomas 1.01–2.00 mm, 28 % in melanomas 2.01–4.00 mm, and 44 % in melanomas exceeding 4.00 mm. However, the correlation between thickness and prognosis is not perfect; occasionally thin melanomas are capable to metastasize, and occasionally patients with thick melanomas have long survival periods.
The Breslow thickness is measured from the top of the epidermal granular layer to the deepest point of dermal invasion. It is important to mention, when present, the involvement of adnexal structures by melanoma in situ. Even though it should not be measured as part of the Breslow thickness, it may result in a positive deep margin by melanoma in situ. At our institutions, the areas of perineural invasion by melanoma are measured. If such measurement is larger than the thickness elsewhere in the tumor, we report the Breslow thickness as including the area of perineural invasion; note this situation in the diagnosis and also provide the thickness of the tumor without including the area of perineural invasion. On the other hand, Breslow thickness should not include areas of vascular invasion or satellitosis. In cases in which there is ulceration, the measurement should be made from the base of the ulcer to the deepest point of invasion. In some cases there is extensive follicular involvement by melanoma and the invasive component is identified only by surrounding those structures; in such cases, the thickness of melanoma should be measured from the central portion of the follicular structure to the adjacent invasive component.
Clark Level
Dr. Clark in 1969 introduced a histopathologic classification of melanoma based on the level of invasion, demonstrating that the level of invasion was closely related to survival. The anatomic levels of melanoma invasion proposed were level I (melanoma in situ), level II (invasion into superficial papillary dermis), level III (invasive melanoma that fills and expands the papillary dermis), level IV (invasion “well” into the reticular dermis), and level V (infiltration into subcutaneous adipose tissue). After this, many studies have compared Breslow thickness and Clark level by multivariate analysis, and the results have shown that Breslow thickness retains a high level of prognostic significance, whereas Clark level does not [44, 47–49]. These results led to the elimination of Clark level from the melanoma staging protocol of the American Joint Committee on Cancer (AJCC) as a stand-alone criterion. In the current classification, Clark level is considered in those stage 1 cases (thinner than 1 mm in Breslow thickness) in which mitotic counts are not available [37].
Radial and Vertical Growth Phase
Most melanomas evolve through a stepwise process of tumor progression that results in histologic changes associated with prognostic information [50–53]. Tumor progression is considered to be the result of sequential acquisition of genetic abnormalities and modulated by host factors in the microenvironment. The histologic criteria of radial and vertical growth phases were first developed by Dr. Clark based on the concept of staged tumor progression and correlation between the histologic features and the survival rate [54]. Melanomas that are diagnosed in the early stage of tumor progression present clinically as patches or plaques and have thus been termed radial growth phase melanomas. Histologically, the radial growth phase is defined as a lesion in which neoplastic melanocytes are mostly present in the epidermis “melanoma in situ” but may be present in the superficial papillary dermis. Such superficially located melanoma cells usually lack the capacity for proliferation in the dermis, i.e., they are not mitotically active and they do not form tumor nests. These non-tumorigenic invasive melanomas typically have the capacity for local persistence and regrowth if not completely excised, and they also have the capacity for progression. At this stage melanoma lesions do not have the capacity for metastasis. In the vertical growth phase (tumorigenic phase), the neoplastic melanocytes have acquired capacity for proliferation in the dermis (which typically occurs vertically, separating from the epidermis, and has therefore been termed the vertical growth phase). Histologically, vertical growth phase is defined as presence of dermal nests that are larger than any nest in the junctional component or when mitotic figures can be identified within the dermal melanocytes. One study showed that the metastatic potential of a melanoma highly correlates with the presence of vertical growth phase [55].
Mitotic Figures
Tumor mitotic rate was recently introduced as a major criterion for melanoma staging and prognosis. Detailed analysis of the AJCC Melanoma Staging Database showed a significant inverse correlation between primary tumor mitotic rate and survival as an independent predictor of survival (as the number of mitoses/mm2 increases, melanoma survival decreases) [37, 56]. Studies have shown that mitotic rate is the second most powerful predictor of survival outcome after tumor thickness in patients with localized melanoma and fourth after the number of positive lymph nodes, age, and tumor ulceration in patients with regional node micrometastases [37, 57]. Due to these, primary melanoma mitotic rate was incorporated into the seventh edition of the AJCC Cancer Staging Manual as a required element for melanoma staging in T1 patients (Breslow thickness smaller than 1 mm). As such, the mitotic rate replaced the level of invasion (Clark) in defining T1 categories (see above). Either ulceration or presence of any mitotic figure per square millimeter in the dermis is used as a primary criterion for defining T1b-stage melanoma. The upcoming 8th edition of the AJCC will modify the pT1 classification to pT1a (<0.80 mm, no ulceration), pT1b (ulceration or 0.8–1.0 mm).
Although there is no universally accepted method for counting mitotic figures in melanoma, the seventh edition of the AJCC Cancer Staging Manual recommends employing the so-called hot spot, to count them in the tumoral areas in the dermis containing the most mitotic figures. In other fields of pathology, the reported mitotic rate is expressed as the number per 10 high-power fields, but since different microscopes have different field sizes, newer protocols recommend reporting the mitotic count per 1 mm2. After detecting the area with the most dermal mitotic figures, with the ×40 lens or at ×400 magnification, the count is then extended to adjacent contiguous fields until examining an area corresponding to 1 mm2 (usually about 4 1/2 high-power fields). If mitotic figures are sparse and randomly scattered throughout the lesion, any mitotic figure is chosen, the field containing it becomes the first field , and then additional contiguous fields are counted to achieve the equivalent of 1 mm2.
Ulceration
The presence of ulceration should be evaluated in every primary cutaneous melanoma. Ulceration is characterized by full-thickness, epidermal defect, evidence of fibrin deposition and neutrophils, and thinning, effacement, or reactive hyperplasia of the surrounding epidermis in the absence of trauma or a recent surgical procedure. Ulceration is an independent prognostic factor for melanoma-associated survival. Survival rates of patients with an ulcerated melanoma are lower than those of patients with a non-ulcerated melanoma of similar thickness [37]. It has been reported that extent of ulceration provides more accurate prognostic information than the mere presence of ulceration [58]. Due to its preeminence in the AJCC staging classification, it is very important to recognize the difference between tumor-related ulceration and ulceration secondary to trauma or recent surgery. Clinical correlation may be required to determine the cause of ulceration [59].
Regression
Regression is the replacement of melanoma cells by fibrosis, melanophages, lymphocytic infiltrate, and telangiectases with or without residual intraepidermal component. The regression changes can range from focal to extensive. The correlation of regression with prognosis is controversial. One possibility for this controversy is that among different studies, there is lack of consensus on the exact definition and measurement of regression. Some studies have defined regression as complete absence of melanoma cells, whereas others have included areas of partial regression and the active phase of host cell interaction with tumor tissue in their measurements of regression. Some studies have reported that the presence of regression indicates a worse prognosis especially in thin melanomas [60–62]. One study analyzed thin melanomas with evidence of regression and demonstrated only one melanoma that metastasized [63]. Another study analyzed a large number of stage I melanomas and although almost half of melanomas 0.75 mm or smaller had regression compared with only 12 % of melanomas exceeding 3 mm, there was no significant difference between mean disease-free survivals. Furthermore, metastases developed in 19.4 % of patients with regressing tumors compared with 29.85 % of patients with non-regressing tumors [64]. At our institutions, we define the presence of extensive regression in cases in which there is regression in more than 50 % of the invasive component . In contrast, the CAP classification uses 75 % as the cutoff between focal and extensive; therefore, our reports include both cutoffs for the evaluation of regression.
Lymphovascular Invasion
Vascular invasion is identified by the histopathological demonstration of melanoma cells within the lumina of blood vessels and/or lymphatics and is generally regarded as a marker of poor prognosis. It is considered a major prerequisite for metastatic spread. Several reports have shown that vascular invasion significantly increased the risk of relapse, lymph node involvement, distant metastases, and death, and its impact on melanoma outcomes was similar to that of ulceration [65–67]. One study analyzed 476 patients with melanoma and found a 5-year survival rate of 27.3 % in patients with vascular invasion versus 76.1 % in those without vascular invasion [68]. Another study analyzed patients with nodular melanoma, of which 15 % had vascular invasion. Vascular invasion was present in more than half of the patients who had lymph node metastases at the time of diagnosis compared with 12 % of the patients without nodal involvement and, similarly, in 75 % of the patients with distant metastases compared with 12 % of the patients without metastatic dissemination. Survival at 5 years in the patients with vascular invasion was 38 % versus 68 % for those without vessel involvement [69]. However, some studies have shown that vascular invasion does not represent an independent factor in predicting prognosis [67, 70–72]. Regarding histologic assessment of vascular invasion, there are potential artifacts such as tissue shrinkage that can result in the false appearance of a vascular space. Thus some studies have evaluated the detection of lymphovascular invasion by IHC and tried to correlate it with metastasis or survival. One study showed that IHC can reliably identify lymphatic vessel distribution and can detect melanoma cells within lymphatic vessels, but it was highly unreliable in predicting melanoma metastasis, since it failed to detect metastatic spread in more than two thirds of patients with regional node metastasis [73]. On the other hand, one study showed that lymphovascular invasion detected by IHC with D2-40 (podoplanin) in melanomas thicker than 1 mm correlated with sentinel lymph node status and survival [71]. Evaluation of vascular invasion is currently being considered for possible inclusion in the AJCC classification.
Perineural Invasion
Perineural invasion is defined as melanoma infiltration of nerve fibers with subsequent extension of the tumor along the surrounding nerves. At our institutions, we record the presence of perineural infiltration in our reports. The inclusion of this piece of information is very important as some types of melanomas, such as desmoplastic, spindle cell, and acral lentiginous, have a high propensity for perineural invasion. In addition, some studies have shown that neurotropism appears to worsen melanoma prognosis. One study analyzed 190 patients with desmoplastic melanoma and 90 patients with desmoplastic neurotropic melanoma. The 5- and 10-year overall survival rates for all desmoplastic melanoma and desmoplastic neurotropic melanoma patients were 75.2 % and 52 %, respectively [74]; thus, neurotropism appears to be a poor prognostic factor. However, since a strict histologic definition of desmoplastic is not universally accepted, it is possible that some of those neurotropic melanomas were actually only “mixed” and not “pure” desmoplastic lesions. Although metastases are less frequent with neurotropic melanomas, they do have high local recurrence rates, which likely derive from their deep infiltration and extension along nerve sheaths [75, 76].
Microscopic Satellitosis
Microsatellites are defined in the current CAP recommendations as microscopic and discontinuous cutaneous and/or subcutaneous metastases having a diameter larger or equal to 0.05 mm in largest dimension, adjacent to a primary melanoma [77]. At our institutions we use a slightly different definition, i.e., clusters of tumor cells separated from the main invasive component by normal dermal collagen or subcutaneous fat. The presence of microsatellites increases from 4.6 % in tumors less than 1.5 mm to 65 % in those greater than 4 mm [77, 78]. Only few studies have evaluated the role of microsatellites as a prognostic factor in cutaneous melanoma. Although it is difficult to predict whether their presence represents an independent prognostic factor, satellites in tumors thicker than 1.5 mm do appear to correlate with a higher risk of local recurrence and with an increased frequency of regional lymph node metastasis [78]. Microsatellites are also associated with an increased frequency of regional lymph node metastasis in tumors greater than 1.5 mm.
Tumor-Infiltrating Lymphocytes
Tumor infiltrating lymphocytes (TILs) are believed to represent the degree of immune response to melanoma cells. They are measured by assessing the extent of lymphocytic infiltrate surrounding the invasive dermal component of melanoma, categorized as brisk, non-brisk, or absent (minimal). The role of TILs as prognostic factors has been suggested by several reports, although with conflicting data [79–81]. These differences may be due to different reasons such as the difficulty to grade a TILs infiltrate using the brisk and non-brisk categories that studies may be underpowered to demonstrate that TILs are an independent prognostic factor or that there is still scarce information regarding the immunology and pathobiology of TILs. The presence of a brisk inflammatory infiltrate has been reported to correlate with improved survival; however, this is observer dependent, mainly owing to the lack of a uniform definition of host response in terms of type and location of the infiltrate. Studies have shown 5- and 10-year survival rates for melanomas with a brisk infiltrate of 77 % and 55 %, respectively, compared to 53 % and 45 % for tumors with a non-brisk infiltrate and 37 % and 27 % for those with absent TILs [82].
Subtypes of Melanoma
The initial classification of primary cutaneous melanoma was based on observations and descriptions of the clinical and histopathological features, which was first described over four decades ago [54, 83]. Lately, there have been some minor changes to the classification scheme, but the basic findings as described over 40 years form the foundation for the currently recognized subtypes of cutaneous melanoma [53, 84]. These authors started by describing the clinical appearance of the lesion, the anatomic site, the presence or absence of sun damage, and the patient’s demographics. All these features were considered alongside the histologic features of the tumors, including intraepidermal and dermal patterns of growth, cytology, epidermal changes, the presence of solar elastosis, the anatomic level of invasion into the dermis, the maximal tumor thickness, vascular invasion, mitotic activity, and the pattern and density of lymphocytic host response. Using these variables, the authors recognized four major subtypes : superficial spreading, lentigo maligna, nodular, and acral lentiginous melanoma.
Lentigo Maligna
Clinical Features
Lentigo maligna was first described by Sir John Hutchinson in 1892 as a “senile freckle .” The Hutchinson melanotic freckle was originally thought to be infectious because of its slow, yet progressive, growth. Some authors refer to it as lentigo maligna when it is confined to the epidermis and lentigo maligna melanoma when it invades into the dermis, but in our opinion, there is no need to distinguish in situ and invasive melanoma with two different names. Lentigo maligna originates on chronically sun-exposed skin, particularly the head and neck although less common sites include the arm, leg, and trunk, in general after chronic exposure to ultraviolet light. Patients with lentigo maligna tend to be older than those with superficial spreading or nodular melanoma. Usually, patients with lentigo maligna are older than 40 years, with a mean age of 65 years and the peak incidence occurs in the seventh to eighth decades of life. The majority of patients with lentigo maligna present with a slowly enlarging, pigmented macule that may remain stable or enlarge slowly or rapidly. Lentigo maligna can be present for long periods, usually between 5 and 15 years, before it invades into the dermis; however, there are reported cases of rapid progression. The risk for progression to invasion appears to be proportional to the size of the lesion of lentigo maligna [85]. The fact that lentigo maligna lacks some of the histopathologic characteristics ascribed to other types of melanoma in situ (particularly pagetoid upward migration and prominence of intraepidermal nests) and may remain stable for years, progress or even regress spontaneously, has led some authors to suggest that lentigo maligna is a precursor or dysplastic lesion rather than being an in situ melanoma [86, 87]. Clinically, individual lesions are characterized by asymmetry, irregular border, heterogeneous color, enlarging diameter, and change in appearance over time. Areas of partial regression within the lesion are not uncommon, and they appear as gray discoloration. It should be recognized also that the clinical extent of lentigo maligna may be difficult to determine based on visual inspection alone. In rare occasions, lentigo maligna can present as a hypopigmented, scaly patch that can mimic squamous cell carcinoma in situ or basal cell carcinoma [88–90]. The clinical diagnosis of lentigo maligna can be difficult particularly in early lesions. The differential diagnosis includes solar lentigo and macular seborrheic keratosis. Dermoscopy and in vivo confocal microscopy have been used to help with diagnosis; however, the gold standard of diagnosis continues to be histopathology [91, 92].
Histopathologic Features
Lentigo maligna goes through different phases. At its early stage, the histologic features of lentigo maligna are subtle and can be confusing due to the apparent bland nature of the intraepidermal melanocytes. Histologically, it is composed of single melanocytes located at the basal layer of epidermis, often with only minimal atypical features (such as mild nuclear enlargement and with or without hyperchromasia). These melanocytes tend to lose polarity of nuclei against the basement membrane, and in some cases, they show a clear halo with a moth-eaten appearance of the nuclei. This bland population of melanocytes is consistently accompanied by solar damage. A diagnostic clue at this early stage is the presence of asymmetric and confluent disposition of melanocytes in the basal layer and the involvement of hair follicles. One needs to keep in mind that the architectural changes may be much more pronounced than the degree of cytologic atypia at this stage. Importantly, smaller atypical melanocytes of lentigo maligna may be partially obscured by surrounding enlarged pigmented basal keratinocytes. Unfortunately, the presence of an early, pigmented actinic keratosis or of a pigmented macular seborrheic keratosis on chronically sun-damaged skin does not preclude concurrent lentigo maligna. The difficulty on making an unequivocal diagnosis at this stage is accentuated especially when the pathologist is dealing with a small tissue sample. As most lesions are located on the head and neck, small biopsies are usually taken to make the diagnosis before definitive treatment is given. Small tissue samples often pose diagnostic difficulty as the biopsy may not be representative of the entire lesion thus resulting in underdiagnosis of lentigo maligna.
As the lesion of lentigo maligna progresses, the histologic features are more pronounced and better appreciated on light microscopy. At this intermediate stage, lentigo maligna shows a confluent lentiginous and sometimes unevenly distributed, nested proliferation of enlarged melanocytes involving the basal layers of the epidermis and extending down appendageal epithelium, again associated with epidermal atrophy and solar elastosis. Melanocytes are commonly hyperchromatic and small with dense nuclear chromatin and unapparent nucleoli. Multinucleated melanoma cells (including “starburst” forms) are often present, but their presence is not specific for lentigo maligna since they can be seen also in benign lesions. There is often a variable superficial dermal inflammatory response with pigment incontinence. In our experience one of the most reproducible histological parameters that is used to confirm a diagnosis is the proliferation of atypical melanocytes at the dermal-epidermal junction in small nests or single cells, with involvement of the skin adnexa and coupled with underlying solar damage. Pagetoid spread of melanocytes is unusual in this type of melanoma and is generally seen later in the progression of the disease, often when there is dermal invasion. The distinction from actinic intraepidermal melanocytic hyperplasia (increased intraepidermal melanocytes secondary to chronic sun exposure) can be exceedingly difficult (also see differential diagnosis below). This reactive condition is also characterized by increased numbers of single basilar melanocytes occurring in the setting of an atrophic epidermis, with diminished rete ridges. The melanocytes tend to be hyperchromatic and slightly enlarged and do not significantly differ from those seen in lentigo maligna. The main distinguishing features are numbers of melanocytes (higher density in melanoma in situ), presence of pagetoid extension (when present in the later stages), and extension down cutaneous appendages. Immunohistochemistry may be helpful to highlight the numbers and size of intraepidermal melanocytes. Since anti-MART-1 is very sensitive, it may prominently label the cytoplasmic dendrites of melanocytes thus resulting in a relatively large area of the epidermis labeled with the immunodye (e.g., DAB), thus resulting in an overdiagnosis of melanoma [93]. HMB-45 is usually less sensitive than anti-MART-1 and thus the area covered by the immunodye is less extensive. At any rate, rather than evaluating the “positive area” highlighted by the dye, it is better to determine how many nuclei are surrounded by immunodye since such quantification will be closer to the actual number of melanocytes present in the epidermis [94].
When lentigo maligna becomes invasive in the dermis, melanoma cells are usually arranged in dermal nests and single cells, and there is frequent vascular ectasia in the superficial vascular. The dermal melanocytes most commonly display an epithelioid or spindle-shaped morphology. Melanocytes are hyperchromatic and atypical, but frequently lack the vesicular nuclei and prominent eosinophilic nucleoli that are seen in other subtypes of melanoma (such as superficial spreading or nodular melanoma). Also, in cases of early invasive lesions with only a few single cells or small nests within a fibrous or inflamed superficial dermis, these invasive cells may be difficult to identify. Immunohistochemistry (IHC) may be used to highlight the focal dermal invasion in cases with only individual invasive melanocytes or small nests admixed with inflammatory cells [94]. The most sensitive immunohistochemistry marker for melanocytes is probably S-100 protein; however, this marker has relatively low specificity, as dermal dendritic cells are positive for S-100, making specific identification of individual melanocytes in the dermis somewhat difficult. Although anti-MART-1 is more specific than anti-S100, it may be still positive in pigmented dermal macrophages [95]. Thus, HMB-45 or antibodies against some nuclear melanocytic markers (MITF or SOX10) may be more helpful in detecting dermal invasion.
As seen in other melanoma subtypes, dermal maturation in lentigo maligna is not apparent, and mitotic figures may be observed, but these are usually few in number. Melanocytes can be found individually throughout the dermis or seen along the skin adnexa. The spindle-shaped melanocytes have a predilection for nerves within the reticular dermis, and perineural invasion is relatively often in lentigo maligna. Some cases of lentigo maligna can be associated with desmoplastic melanoma, and the invading spindle tumor cells are very subtle, bearing a striking resemblance to a dermal scar. In such cases, anti-S100p, anti-MITF1, or anti-SOX10 is very helpful to make such distinction (since other markers such as MART-1 or HMB-45 antigen are often negative in desmoplastic melanoma).
Differential Diagnosis
Lentigo maligna has traditionally been difficult to diagnose, especially in its early stage or when a limited tissue sample is provided. Recent studies about the molecular pathogenesis of melanoma revealed that melanomas occurring on the sun-exposed skin show a distinct pattern of chromosomal instability sometimes related to UV DNA damage and are less frequently associated with BRAF mutations than superficial spreading melanoma (i.e., occurring on intermittently sun-exposed skin).
The main differential diagnosis of lentigo maligna is with lesions associated with sun damage such as early lesions of seborrheic keratosis, lichen planus-like keratosis, pigmented actinic keratosis/solar lentigo, and junctional melanocytic nevus. However, differentiating lentigo maligna from a background of increased melanocytes on chronically sun-damaged skin is one of the most difficult diagnostic challenges in diagnostic dermatopathology. In its earliest stages, lentigo maligna shows a single-cell, lentiginous proliferation of atypical melanocytes at the dermal-epidermal junction with only minimal cytologic atypia. It is well known that benign reactive melanocytic hyperplasia in sun-exposed areas or in the vicinity of surgical scars can exhibit histologic patterns similar to early lentigo maligna. In fact, the classic morphologic depiction of chronic photoactivation of melanocytes includes lentiginous melanocytic proliferation with the potential for nuclear hyperchromasia, binucleation and multinucleation, and low-level pagetoid ascent. Thus, a proliferation of melanocytes with slightly atypical nuclei in skin severely UV damaged is not sufficient for a diagnosis of lentigo maligna. Sun-exposed epidermis shows approximately twice as many melanocytes as non-sun-exposed, covered skin, and the density of melanocytes has been said to be proportional to cumulative sun exposure. As the lesion of lentigo maligna progresses, the histologic changes are more obvious, including coalescence of single melanocytes along the dermal-epidermal junction, extension into skin adnexa, focal pagetoid spread, and obvious junctional nest formation. Indeed, a very important and useful feature for the diagnosis of lentigo maligna is the presence of intraepidermal melanocytic nests. Furthermore, some authors have suggested that, if there are melanocytic nests in an entirely intraepithelial lesion on the skin in the head and neck area with severe solar elastosis, the diagnosis almost always is lentigo maligna. However, we consider that patients may develop junctional dysplastic nevi during all their life, and therefore some may occur on the face after sun damage has started.
The in situ component of lentigo maligna is usually wide and poorly circumscribed, with individual neoplastic melanocytes extending beyond the last melanocytic nest; thus, the absence or presence of melanocytic nests does not always help differentiate lentigo maligna from melanocytic hyperplasia in sun-damaged skin and is only rarely helpful in delineating the borders of a melanoma. The distribution of pigment is relevant in distinguishing these two conditions, as in cases of solar lentigo with melanocytic hyperplasia, there is an even distribution of melanin in basal keratinocytes, as opposed to the irregular distribution of pigment noted in lentigo maligna [96]. An important diagnostic clue is the presence of preserved elongation of rete ridges that are relatively uniform and relatively equidistant from each other in cases of solar lentigo/pigmented actinic keratosis, in contrast to lentigo maligna in which the rete ridges tend to be flattened. In cases of lentigo maligna in which there is focal preservation of rete ridges, they tend to be usually irregular and not equidistant from one another.
As mentioned above, immunohistochemistry plays an important role to separate incipient lentigo maligna from intraepidermal melanocytic hyperplasia on sun-damaged skin. Mere close inspection of H&E-stained section does not always allow an unequivocal diagnosis, because it is sometimes difficult to distinguish pigmented keratinocytes from melanocytes. Anti-MART-1 may overestimate the number of melanocytes because it labels the melanocytic dendrites and might also label pigmented keratinocytes, including structures mimicking junctional melanocytic nests in the setting of a lichenoid infiltrate [97–99]. This lack of specificity may result in false-positive result in the assessment of difficult intraepidermal melanocytic lesions. Both MITF-1 and SOX10 are nuclear makers that facilitate the diagnosis [100] since they avoid the cytoplasmic labeling seen with anti-MART-1 or HMB-45.
A similar challenge may occur when evaluating the peripheral margins of a melanoma in situ in a re-excision specimen, since epidermal melanocytes are usually increased in number as a reaction to the prior trauma (biopsy). Among the criteria that can be used for delineating the borders of a melanoma are presence of melanocytes above the junction, pleomorphism or hyperchromasia of melanocytes, and a high density of melanocytes with a confluent pattern.














Fig. 7.1
Early lentigo maligna melanoma in situ. This lesion is located in the forehead of a 53-year-old male. The lesion is composed of a subtle increase of intraepidermal melanocytes accompanied by flattening of the epidermis and diffuse solar elastosis (a). Single-cell proliferation of melanocytes in the epidermis with focal extension into skin adnexa. As characteristically observed in lentigo maligna lesions, there is no prominent pagetoid extension (b). Increased numbers of large-sized melanocytes along the dermal-epidermal junction and into a hair follicle (c). Early note the large size of melanocytes with visible nucleoli. Single nest (right) (d)

Fig. 7.2
Lentigo maligna melanoma in situ. This lesion is located on the neck of an 83-year-old male. Flattening of rete ridges and solar elastosis (a). Diffuse and confluent proliferation of melanocytes along the dermal-epidermal junction (b). Higher power highlights the increased number of melanocytes in the epidermis. There is follicular extension of the atypical melanocytes (c)


Fig. 7.3
Lentigo maligna melanoma in situ. This lesion is located in the scalp of a 70-year-old male. Depending on how the lesion is sampled, it can be difficult to interpret (specially in small biopsies, where the edge of the lesion can simulate intraepidermal melanocytic hyperplasia in sun damage) (a). On low magnification, there is an abnormal effacement of the epidermis along with prominent solar elastosis (b). Confluent single-cell melanocytes without nest formation (c). The melanocytes have prominent nuclear pleomorphism (d)


Fig. 7.4
Lentigo maligna melanoma in situ. In contrast with Figs. 7.1–7.3, in this example the lesion is more asymmetric, with areas of epidermal hyperplasia and focal host response (a). There is effacement of the rete ridges and isolated vacuolated cells (melanocytes) at the dermal-epidermal junction (b). Nests and single cells in the epidermis. Note the areas of fibrosis, inflammation, and pigment incontinence in the superficial dermis (c). High-power view of large, hyperchromatic melanocytes at the dermal-epidermal junction (d)

Fig. 7.5
Lentigo maligna melanoma in situ, with adnexal extension. There is diffuse proliferation of melanocytes along the dermal-epidermal junction (a). The follicular structures are involved by atypical melanocytes. An important point to remember (specially in small biopsies) is that intraepidermal melanocytic hyperplasia induced by chronic sun damage can also involve the adnexal structures but usually only involve the follicular infundibulum and acrosyringia. Note in this case that the extension of the hair follicles is deep (b, c)


Fig. 7.6
Solar lentigo with intraepidermal melanocytic hyperplasia. This is a 56-year-old female that presented with a light pigmented lesion on her cheek (a). The melanocytes are present in single units, however, distant from each other and without a confluent growth (b). Anti-MART-1 shows increased number of melanocytes but without a confluent growth (equidistant melanocytes) (c). Higher-power view of MART-1 expression showing melanocytes as single units (not nested or confluent) (d)


Fig. 7.7
Lentigo maligna melanoma of the ear associated with a congenital nevus. This case is located in the ear of a 61-year-old male. The patient had a nevus in this location and recently the lesion increased in size, shape, and color (a). The image depicts an intradermal nevus with congenital pattern, and the overlying epidermis shows a confluent growth of melanocytes (more prominently seen in the hair follicle (arrow)) (b). The nevus cells in the dermis show periadnexal pattern, consistent with a congenital onset (c). Note the characteristic confluent pattern of growth of melanocytes along the dermal-epidermal junction and skin adnexa (d)
Superficial Spreading Melanoma
Clinical Features
The term superficial spreading melanoma was first coined by Dr. Clark, as a melanoma that grows primarily on the trunk and proximal extremities, with a nested, intraepidermal pattern of growth and prominent pagetoid upward migration. This type of melanoma accounts for around 70 % of all melanomas. The prevalence of superficial spreading melanoma has increased in the last few years. Apart from a possible connection with increased exposure to UV light, it is possible that this increase may be due to a lower threshold for its diagnosis. It has been suggested that many cases that were previously identified as dysplastic nevi are now being classified as melanomas [101]. The first clinical sign is the appearance of a flat or slightly raised discolored, asymmetrical patch that has variable pigmentation and with irregular and indurated borders. The color varies, with areas of tan, brown, black, red, blue, or white, with sometimes protruding blue-black papules/nodules. The histologic correlates of these variations in color are the presence of dermal inflammation, regression, and the deep dermal melanin pigment in macrophages. Small, notch-like indentations may be noted at the edge of the lesion. Ulceration is sometimes seen, a finding rarely seen in nevi and also associated with impaired prognosis. Lesions are usually larger than 10 mm (average 15 mm), but in rare occasions superficial spreading melanoma can present as a smaller lesion . Superficial spreading melanoma can be found almost anywhere on the body, but is most likely to occur on the trunk in men, the legs in women, and the upper back in both.
Histopathologic Features
The in situ component of superficial spreading is characterized by an asymmetrical proliferation of atypical melanocytes scattered singly and in clusters throughout all levels of the epithelium giving an appearance reminiscent of Paget disease (pagetoid upward migration or “buckshot” scatter). These pagetoid melanocytes are scattered throughout the epidermis giving a moth-eaten appearance and sometimes even infiltrating the stratum corneum. Melanocytes are epithelioid in appearance with abundant cytoplasm, often showing fine or dusty melanin pigmentation, and pleomorphic vesicular nuclei with prominent, eosinophilic nucleoli. There may be necrotic melanocytes and scattered mitotic figures including atypical forms. Melanocytic nests within epidermis tend to be irregularly distributed and have a confluent growth. An important diagnostic clue is that the amount of cellularity, pigment, and size of melanocytes vary from to nest. These melanocytic nests are large in general and have irregular contours; in occasions this pattern predominates over pagetoid melanocytes. Most of such cases of superficial spreading melanoma composed predominantly of large nests have only scant intraepidermal or junctional single melanocytes; thus, histologic recognition can be difficult [102, 103]. “Consumption” of the epidermis is present in approximately half of cases of superficial spreading melanoma. This epidermal consumption is defined as thinning of the epidermis with attenuation of basal and suprabasal layers and loss of rete ridges adjacent to collections of melanocytes. The epidermis may become separated from the dermis by an artefactual cleft (“zipper” sign; Christopher R. Shea, personal communication). Some authors believe that this consumption of the epidermis appears to represent a precursor to ulceration. In contrast to lentigo maligna, there is often little visible evidence of actinic damage. Also, in some cases there is lymphocytic infiltration in the papillary dermis and this may herald the presence of rare invasive cells. There may be fibroplasia of the papillary dermis; however, the organized (lamellar or concentric) stromal response observed in dysplastic nevi is not seen in such cases.
As per the original description by Dr. Clark, extension of the intraepidermal component for more than three rete ridges past the dermal component is a must to classify the lesion as superficial spreading (or acral lentiginous or lentigo maligna) melanoma. Progression of the radial growth phase starts with the development of a microinvasive component as single cells or small nests in the papillary dermis. These melanocytes have a similar cytomorphology as the intraepidermal component. When these dermal melanoma cells display either nests larger than observed in the overlying epidermis or have mitotic figures, these are the features of vertical growth phase. The invasive component in the dermis may be arranged in single melanocytes and solid nests or may have a fascicular growth pattern. Melanocytes may be epithelioid, spindle-shaped, small (nevus-like), and vacuolated (balloon cell-like). These melanocytes are pleomorphic and have irregular nuclei with irregular, hyperchromatic chromatin and thick nuclear membrane. The nuclei have an irregular outline that can be indented. The melanocytic density in melanomas is high, and the cells seem to overlap one to another. Melanocytes can form expansile nodules, which usually compress the adnexal structures. The presence of irregular distribution of pigment in the dermis is another diagnostic clue of melanoma. Detection of dermal mitotic figures in melanoma is a very important finding to confirm the diagnosis; however, absence of dermal mitotic figures should not be considered sufficient to exclude a diagnosis of melanoma. Particularly relevant is the presence of mitotic figures in the deeper portion of the lesion, since such a finding is only exceptionally seen in nevi. Recent studies have used immunodetection of phosphohistone H3 to facilitate the identification of mitotic figures in various neoplasms, including melanomas [104–106]. One study in particular provided definite support for the application of MART-1/phosphohistone H3 immunohistochemistry as an adjunctive test in the evaluation of primary melanomas [107]. This study concluded that adding this ancillary test could potentially add important value to the analysis and characterization of primary cutaneous melanoma, especially this is particularly true for the description of thin melanomas (≤1.0 mm) where mitotic figures can be difficult to identify and for which the identification of a mitotic figure would have the most profound implications for staging and patient management. In our practice, we apply dual immunohistochemical staining for MART-1 and phosphohistone H3 in cases where mitotic figures are seen in the infiltrate, but it is unclear if they represent melanocytes or other cells (e.g., macrophages, endothelial cells) or it is unclear if a cell is on mitosis or apoptosis.
Differential Diagnosis
Superficial spreading melanoma can mimic other melanocytic and non-melanocytic neoplasms. The main melanocytic lesion that needs to be considered in the differential diagnosis of melanoma is dysplastic nevus. In occasions, the distinction can be difficult and somewhat subjective, especially if the dysplastic nevus has severe architectural and cytologic features. Features that favor melanoma over a dysplastic nevus are the presence of significant pagetoid upward migration especially at the lateral borders of the lesion, diffuse and even cellular atypia, and the presence of deep-seated mitotic figures in the dermal component (see also the dysplastic nevus chapter). Another important clue is the presence of confluent nests in the dermal-epidermal junction, as it tends to abut the undersurface of the epidermis in between the rete ridges. In the differential diagnosis between melanoma and dysplastic nevi, it must be considered that focal areas of pagetoid upward migration in a nevus can be a sign of melanoma in situ originating in a dysplastic nevus. On the other hand, trauma to a nevus can induce focal pagetoid upward migration and this should not be interpreted as melanoma in situ. An important feature to distinguish between focal melanoma and prior trauma in a nevus is the presence of pigmented parakeratosis in the latter. Both melanoma and dysplastic nevus may show regression; however, in dysplastic nevi regression is usually focal as opposed to cases of melanoma in which the regressive changes are usually more widespread.
Recurrent/persistent nevi may simulate superficial spreading melanoma. The most important piece of information to make such distinction is to review the previous specimen. Histologic features that favor nevus include the sharp circumscription of the lesion, as recurrent nevi tend to preserve circumscription as opposed to melanomas, which are asymmetric. A banal intradermal component supports the diagnosis of recurrent nevus, as in melanomas the dermal component shows an atypical growth along with the presence of deep mitotic figures. In recurrent nevi, the intraepidermal growth is above the scar, while the epidermis beyond the scar is uninvolved. The presence of pagetoid upward migration beyond the area of scar in the dermis is almost always diagnostic of melanoma.
Non-melanocytic skin lesions can also mimic superficial spreading melanoma. Merkel cell carcinomas can be easily confused with melanoma, especially in superficial shave biopsies, since they can show an intraepidermal growth with marked pagetoid upward spread and forming small nests. If the tissue sample allows inspection of the dermis, it will become obvious the characteristic round blue cell morphology of Merkel cell carcinoma. Immunohistochemistry will allow easy recognition of both of these entities (keratin and CK20 in Merkel cell carcinoma, both unlikely to be seen in melanoma). Paget and extrammamary Paget disease can also mimic superficial spreading melanoma. Both of these conditions form nests and single cells with pagetoid growth in the epidermis and thus can look almost identical to superficial spreading melanoma. Cells in Paget disease tend to be located above the dermal-epidermal junction (so-called “eyeliner” sign ). Paget cells express keratins, including CK7, and only rarely can they express S100 (other melanocytic markers are consistently negative in Paget disease). Examples of metastatic gastrointestinal, prostate, and lung carcinoma can occasionally invade the epidermis and thus can easily simulate melanoma. In general such lesions have widespread vascular invasion or glandular arrangement. Also, immunohistochemistry will allow a more specific distinction depending on the type of lesion. Epidermotropic metastatic carcinomas can mimic superficial spreading melanoma; in such cases, clinical-pathologic correlation will be essential (i.e., history of prior melanoma of similar morphology and located nearby).













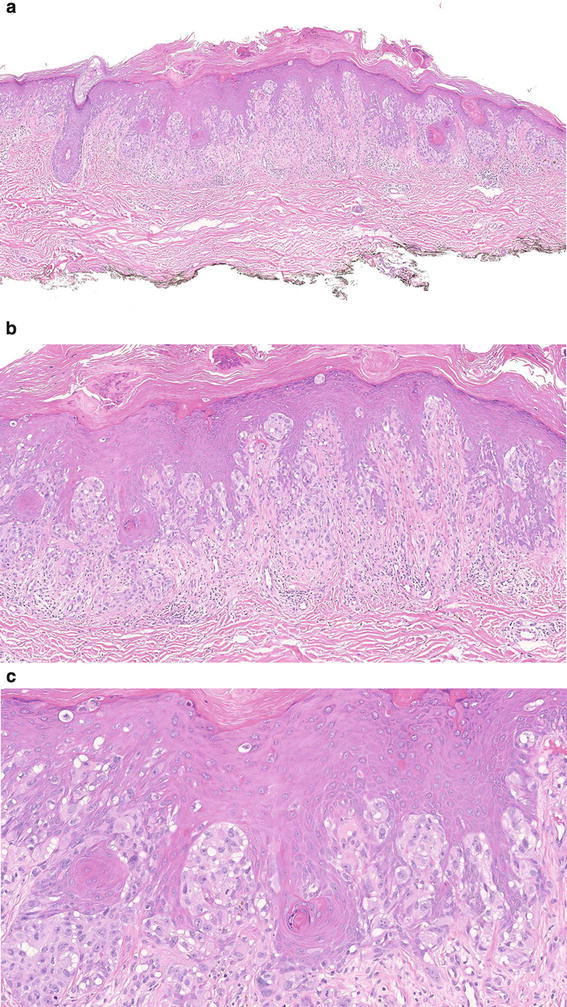



































Fig. 7.8
Superficial spreading melanoma in situ. This case is composed of an irregular junctional growth of melanocytes mostly aligned in the dermal-epidermal junction in single cells (a, b). The melanocytes are epithelioid and display a single-cell pattern in which they are close together and in some other areas they are far apart. There is increase pagetoid upward migration throughout the lesion (c). The cells have variable nuclear pleomorphism and irregular thick membrane and are surrounded by clear halos (d)


Fig. 7.9
Lentiginous superficial spreading melanoma in situ. This is an example of superficial spreading melanoma in which the cells have a predominant lentiginous growth (a). The low-power image shows a lentiginous nevus-like growth; however, the degree of cellular density and the asymmetry of the lesion are clues to the diagnosis of melanoma (b). On higher magnification, the lesion shows atypical nests along with an increased number of atypical melanocytes with prominent pagetoid upward migration (c, d)

Fig. 7.10
Superficial spreading melanoma in situ with adnexal extension. This example shows a clear melanoma composed of single and nested melanocytes seen at the bases and the flanks of the rete ridges (a). On low magnification, one can see the irregularly shaped nests (b). This example shows extensive adnexal extension. On higher magnification, the melanocytes have an ample pale cytoplasm with pleomorphic nuclei (c)


Fig. 7.11
Superficial spreading melanoma in situ with adnexal extension and prominent host response. This lesion is composed of atypical bizarre-shaped nests with increase confluent of growth (a). Areas with early epidermal consumption are noted (b). The lesion shows extensive adnexal extension and increased host response in the dermis (c). Areas of superficial invasion are noted in the papillary dermis (d)


Fig. 7.12
Superficial spreading melanoma in situ. This example shows on low magnification a dysplastic-like architecture, including bridging of rete and fibroplasia of the papillary dermis (a, b). However, one can clearly identify the increase density of melanocytes in the dermis along with increase number of atypical epithelioid melanocytes with prominent pagetoid spread throughout the thickness of the epidermis. Note the atypical melanocytes involve the rete ridges not only at the tips but the lateral sides and the suprapapillary plates (c, d)


Fig. 7.13
Superficial spreading melanoma with superficial invasion. This example displays a lesion that is primarily composed of nests in the epidermis. Note variable size and shape of nests with areas. Areas with pagetoid growth (a, b). The cells are round, with pleomorphic nuclei, and have a characteristic ample pale cytoplasm. Note that early invasion is seen in the papillary dermis (c, d)


Fig. 7.14
Invasive superficial spreading melanoma. This example shows areas of clear invasion along with areas of early regression. The in situ component of the lesion has a single-cell growth with nested formation and pagetoid growth. In the dermis, the atypical melanocytes involve the reticular dermis (a, b). On high magnification, the melanocytes in the dermis are pleomorphic, lack maturation, and have similar morphology to those seen in the overlying epidermis. There are early areas of regression composed of pigment incontinence, angiogenesis, and minimal dermal fibrosis (c, d)

Fig. 7.15
Superficial spreading melanoma with pseudoepitheliomatous hyperplasia. This example shows epidermal hyperplasia (which is not unusually seen in melanoma) along with an atypical growth of melanocytes in both the epidermis and dermis (a, b). The cells in the epidermis have a single-cell growth with pagetoid growth. The cells in the dermis are reminiscent to those seen in the epidermis. The melanocytes are epithelioid in shape and display an ample eosinophilic cytoplasm (c)


Fig. 7.16
Ulcerated superficial spreading melanoma composed of epithelioid pigmented and nonpigmented melanocytes. This example of invasive melanoma shows ulceration, but the viable epidermis shows a massive growth of atypical intraepidermal melanocytes with pagetoid growth (a, b). The dermal component shows a biphenotypic component. Most of the dermal component is composed of lymphocyte-like melanocytes with a small to medium hyperchromatic nuclei; however, the center of the lesion shows pigmented epithelioid cells with ample dusky to pale cytoplasm with prominent nuclear pleomorphism (c, d)


Fig. 7.17
Superficial spreading melanoma associated with nevus. This example shows melanoma in situ composed of atypical intraepidermal growth composed of single and nested melanocytes with pagetoid growth. In the dermis, there is a nevus that is cytologically different from the growth in the overlying epidermis (a, b). Per the patient, he observed a pigmented lesion on his arm since he can remember but recently the lesion has grown in size. Note that the melanocytes in the dermis show no cytologic atypia and normally mature toward the base. Also, the dermal stroma surrounding the dermal melanocytes shows normal collagen without the presence of desmoplasia or fibrosis (which is commonly seen in invasive melanomas) (c, d)

Fig. 7.18
Superficial spreading melanoma with dermal regression. This case shows a melanoma in situ along with dermal fibrosis and pigment incontinence (a). Also, note the early vacuolar damage of the epidermis with rare dyskeratotic cells which is not unusually seen in regressing melanomas (b). The overlying epidermis displays severe cytologic atypia of the melanocytes along atypical nests that have confluent growth and single-cell melanocytes with pagetoid upward migration (c)


Fig. 7.19
Superficial spreading melanoma with lichenoid tissue reaction. This is a challenging case as it can mimic lichenoid keratosis or lichenoid dermatitis. Note the epidermal acanthosis, thickened granular layer, vacuolar alteration of the dermal-epidermal junction, and clear lichenoid inflammation in the dermis (a, b). After careful inspection, one can identify the atypical population of melanocytes that is located at the edge of the biopsy and forming an atypical confluent growth of intraepidermal melanocytes (c, d). Remember that lichenoid keratosis can show pseudomelanocytic nests; however, in this example the nests are larger and broader than those seen in cases of lichenoid keratosis. In cases like this, immunostains (MART-1, SOX10, or MITF-1) are an important ancillary test as it can help to delineate the atypical junctional component (keep in mind that pseudonests in lichenoid keratosis can also be highlighted with immunostains)


Fig. 7.20
Superficial spreading melanoma with lichenoid tissue reaction. This example also shows a lichenoid inflammatory reaction in the dermis (a, b). Note the subtle epidermal component that shows atypical melanocytes in a confluent growth along with focal pagetoid upward migration (c). Note the presence of scattered dyskeratotic cells and vacuolar damage of the dermal-epidermal junction. MART-1 is helpful, as it delineates clearly the atypical population of melanocytes within the epidermis (d)


Fig. 7.21
Superficial spreading melanoma with epithelioid melanocytes. This lesion is primarily composed of epithelioid melanocytes. The epidermis shows scattered nests with single pagetoid melanocytes (a). Most melanocytes have an epithelioid morphology with clear/pale cytoplasm, severe cytologic atypia, and nuclear pleomorphism (b). The dermal component shows similar cytomorphology (c). The epithelioid melanocytes have a dusky cytoplasm (d)


Fig. 7.22
Superficial spreading melanoma with invasive nevoid component. This a challenging case, as on low power the lesion is quite subtle (a). At medium magnification, one can identify the increase number of intraepidermal melanocytes in a single-cell pattern without nest formation (b). The intraepidermal growth is subtle; however, there is prominent pagetoid growth throughout all levels of the epidermis (immunostains can be helpful in such cases, as it delineates clearly the atypical melanocytes). There is a small invasive component in which the melanocytes have a nevoid growth; however, there is lack of maturation and presence of enough cytologic atypia to interpret this as invasive component (c, d)


Fig. 7.23
Superficial spreading melanoma. This case shows on the left side of the image an in situ component and the right side shows epidermal hyperplasia with invasion (a). The invasive component shows atypical melanocytes with epithelioid cytomorphology (b). The epidermis overlying the invasive component does not show a clear in situ growth (c, d)


Fig. 7.24
Superficial spreading melanoma with balloon cells. The lesion shows characteristic features of melanoma including the atypical intraepidermal growth, including nests with heterogeneous size and shape (a). The lesion also shows areas of clear epidermal consumption. Many of the nested and single melanocytes have balloon cell melanocytes (cells with ample and bubbly cytoplasm) (b–d)


Fig. 7.25
Superficial spreading melanoma with extensive balloon cell changes. This is a 45-year-old male with a lesion on his arm; the patient has a prior history of melanoma. This is an extraordinary case of melanoma in situ composed of balloon cell melanocytes. One can clearly identify the atypical intraepidermal growth with massive confluence, pagetoid growth, and extension down the adnexa (a, b). Note that all melanocytes have an ample, watery clear cytoplasm with bubbly appearance (c, d)


Fig. 7.26
Superficial spreading melanoma composed of spindle melanocytes. The lesion is located in the trunk of an 84-year-old male. This is an example of in situ melanoma composed primarily of spindle cells. The lesion is composed of an atypical confluent growth of melanocytes within the epidermis (a). The melanocytes are spindle and arranged in confluent nests that are parallel located to the epidermis (b). On high magnification, the spindle cells have only minimal cytoplasm and there is nuclei overlapping with marked pleomorphism. Areas of epidermal consumption are noted (c, d)


Fig. 7.27
Superficial spreading melanoma composed of spindle melanocytes. This case shows minimal in situ component composed of scattered single irregular melanocytes. In the dermis, prominent sheets of melanocytes underneath the consumed epidermis are seen. The solid growth pattern in the dermis shows a fascicular growth (a, b). The spindle cell melanocytes have an eosinophilic cytoplasm, severe cytologic atypia, and a large prominent nucleoli. This case showed scattered atypical mitoses that were easily identifiable (including the base of the lesion) (c, d)


Fig. 7.28
Recurrent superficial spreading melanoma. This patient had a prior history of invasive melanoma s/p excision with clear margins and after 2 years develops this pigmented lesion in the same anatomic site. This case shows an old scar with a clear intraepidermal atypical population of melanocytes with a chronic lymphocytic infiltrate in the dermis (a). The epidermis overlying the scar shows loss of the rete ridge architecture (b). One should keep in mind that a recurrent nevus might show similar features; however, this case shows changes that override a nevus including the predominant single-cell population of melanocytes and severe cytologic atypia (that is not seen in recurrent nevi) that is located next to the area of the scar in the dermis (c, d). Remember that recurrent nevi usually show pseudomelanoma changes in areas that overlie the scar; however, these changes are not seen beyond the scar


Fig. 7.29
Melanoma in situ associated with a dysplastic nevus. This lesion is located on the back of a 68-year-old male. The lesion is composed of elongated rete ridges with spindle and epithelioid melanocytes arranged in a horizontal pattern (a). Note that the nests connect the retes as observed in dysplastic nevi. However, the lesion shows a degree of cytologic that is not accepted for a nevus along with some pagetoid spread of melanocytes (b). The cytology of the melanocytes is quite striking and displays severe cytologic atypia with nuclear pleomorphism (c, d)


Fig. 7.30
Invasive melanoma associated with a dysplastic nevus. This lesion has two components: one is located at the far left of the image and shows a clear invasive melanoma and the second component is located in the center at the far right of the image and shows a dysplastic nevus (a). The melanoma displays characteristic changes and clearly blends with the adjacent dysplastic nevus (b, c). We interpret this lesion as melanoma arising in a dysplastic nevus. The dysplastic nevus shows shouldering and bridging of melanocytes (d)


Fig. 7.31
Incipient melanoma in situ associated with dysplastic nevus. The low-power architecture of this lesion is that of a dysplastic nevus (a). However, in some areas the degree of single-cell proliferation is beyond to what we expect to see in a dysplastic nevus (b, c). Note the single-cell melanocytes that extend throughout all layer of the epidermis (d)


Fig. 7.32
Superficial spreading melanoma with microsatellite nodule. This is an example of invasive melanoma along with a microsatellite nodule in the dermis (a). The dermal nodule is separated from the invasive component by the normal dermis (b). Also, the cytology of the cells in the microsatellite nodule is different from the invasive component. It shows cells with higher degree of cytologic atypia (c)
Acral Lentiginous Melanoma
Clinical Features
Acral lentiginous melanoma (ALM) was first coined by Reed et al. in 1976. It occurs on acral sites, under the nail, and in mucosal surfaces. The overall incidence of melanoma in dark skin patients is low compared with whites; however, ALM makes up a much higher proportion of melanoma in dark skin patients such as in Asians, Hispanics, and African Americans. Hispanic populations seem to have the highest proportion of ALM, when compared with other types of melanoma. Although initially thought to be rare in whites, the difference is more a matter of relative frequency when compared with other types of melanoma occurring in areas continuously or intermittently exposed to sunlight. ALM is the least frequent of the four major histologic subtypes of melanoma overall and accounts for about 2–3 % of all melanomas [108]. The most common location of ALM is the palmar, plantar, and subungual skin. It usually presents as a pigmented macule or papule with irregular borders and variegated pigmentation on the palmar and plantar regions. ALM has a slight female predominance and presents most often in the elderly; however, in white patients ALM tends to have equal incidence in both sexes. Not all melanomas on volar and subungual skin are of acral lentiginous type, some being of superficial spreading, nodular, or unclassifiable type. It has been suggested that a diagnosis of ALM is generally associated with a relatively poor prognosis since tumors are generally thick by the time of diagnosis.
Mucosal melanomas of the oral cavity and other anatomic sites, such as the vagina or rectum, have been included in this histologic group as they share some clinical, histological, and molecular similarities with ALM. The predilection of ALM for plantar locations has led many authors to believe that trauma may play an important role in the etiology of ALM, since it does not appear that sun exposure plays a significant risk factor for ALM [109, 110].
Clinically, ALM is easier to identify clinically in advanced stages as it usually presents as irregular, asymmetric pigmented macules that can be several centimeters in diameter. As ALM progresses to vertical growth phase, it frequently ulcerates. All clinicians and pathologists should be aware of the importance of the diameter as a critical factor when diagnosing ALM. Many studies have clearly defined that acral melanocytic lesions that are large (>7 mm in diameter), flat, and heavily pigmented are almost always ALM, even considering the subtle changes seen in some areas of such lesions [111]. Also, when ALM develops a vertical growth phase, it can mimic a nodular melanoma (see below). Clinically, nodular melanoma on acral sites is very rare, and when such a pattern is seen, it is most likely the vertical component of a flat lesion that went misdiagnosed for many years. Unfortunately, amelanotic forms of ALM can be exceedingly difficult to identify, and in such cases, the disease may go unsuspected even by experienced clinicians.
Histopathology
One of the major challenges in dermatopathology is to differentiate acral junctional nevus versus ALM in situ. Making a diagnosis of invasive melanoma on acral locations is not difficult; however, the diagnosis of acral lentiginous melanoma in situ can be quite difficult as it can mimic junctional nevi.
The early stage of ALM (in situ component) is composed of single melanocytes that are irregularly distributed along the dermal-epidermal junction. Intraepidermal nests are often absent at this stage and some lesions can even grow and reach a large size without forming nests. As the lesion evolves, nests can be observed, and when noted they are irregular in size and shape, are elongated, and are located parallel to the epidermis. Such nests show lateral confluence and spindle cell configuration. A characteristic feature when nests are present is that they alternate with single melanocytes forming skipping areas (in some cases one can notice areas that lack melanocytes altogether). Melanocytes tend to be elongated, plump, with darkly pigmented nucleus, and a surrounding halo. Dendrites are commonly visible, highlighting the presence of migrating melanocytes into mid- and higher levels of the epidermis. These dendrites are irregular in thickness, have different lengths, and have a tendency to form a web around the basal cells. Melanocytes with ample and dusky cytoplasm are rare but pagetoid spreading is not uncommonly observed. These pagetoid spread is not as noticeable as in other anatomic locations, mainly because of the small size of the nucleus in the melanocytes. Also, it is common to observe a cleft (“zipper” sign) at the dermal-epidermal junction in areas where basal melanocytes overwhelm the number of basal keratinocytes. Pigment is present irregularly along the stratum corneum (as opposed to being in columns characteristic of acral nevi). Early examples of ALM can be identified by the presence of individual melanocytes crowding around the crista profundae intermedia, especially if melanocytes are also detected along the junction between the cristae. This stage can be a diagnostic challenge as melanocytes display only minimal cytologic atypia. It is not unusual to identify melanocytes that extend irregularly into the eccrine ducts. This extension into the eccrine duct differs from that of nevi, as in nevi one can see melanocytes gathered in and around ducts forming well-defined nests as in ALM melanocytes are irregularly distributed and in a single-cell pattern. In some cases of ALM, this syringotropic spread of malignant melanocytes can reach the deep portion of the duct, and thus melanoma in situ may be present at the deep margin. The epidermis is usually acanthotic, hyperplastic, and hyperkeratotic. At this in situ stage, there may be a dermal lymphocytic infiltrate, a feature usually absent in acral nevi).
Experts in the topic have proposed that ALM in situ should be considered a clinicopathologic entity [112]. These authors have proposed that when a classic clinical picture of ALM is seen, e.g., irregularly shaped, darkly pigmented, flat lesion with a diameter >7 mm, pathologists should strongly suggest the diagnosis of ALM in situ if the histology shows a proliferation of single melanocytes along the dermal-epidermal junction.
Usually after years, the lesion, once limited to the epidermis, becomes infiltrative into the dermis. The overlying epidermis still shows the atypical lentiginous pattern and at this stage is more common to observe a nested component. The nests are irregular in size and shape and have a confluent pattern of growth and areas of epidermal “consumption.” The dermal component can show either spindle or epithelioid melanocytes (more commonly spindle). The atypical features become more obvious, and a clear diagnosis of melanoma can be easily made. When ALM is already invading in the dermis, the overlying epidermal component can sometimes be limited to an inconspicuous lentiginous melanocytic proliferation as seen in the very early stage of ALM in situ (macular, in situ component of the invasive neoplasm).
Differential Diagnosis
Acral skin has peculiar anatomic aspects that are important to keep in mind when evaluating acral melanocytic lesions. Rather than flat surface, it is composed of ridges and furrows, as seen in fingerprints. Ridges are wider than furrows and at their center there is an opening of the eccrine ducts. This particular anatomic feature of the acral skin often causes nevi in these locations to adopt a striped arrangement; thus, this is very important to evaluate when inspecting acral nevi. It has been recommended to cut the microscopic sections of any melanocytic lesion on acral skin perpendicular to the furrow pattern, to allow a better appreciation of the symmetry and circumscription of the lesion. The pattern most commonly seen in acral nevi is that of individual melanocytes concentrating at the base of the furrows and sparing the areas at the base of the ridge around the acrosyringium.
The clinical history is important when evaluating acral melanocytic lesions; acral melanomas are almost always seen in older patients, whereas acral melanocytic lesions in younger patients are almost always benign nevi. The early phase of ALM in situ can be very difficult to distinguish from acral nevus, especially in small samples. When dealing with small, pigmented lesions on acral locations that are clinically suspicious for ALM, clinicians should consider an excisional biopsy with a narrow margin. ALM in situ tends to have a lentiginous growth pattern with rare nest formation (seen in more advanced stages) with melanocytes that are angulated and with hyperchromatic nuclei. When junctional nests are seen, they are irregular in shape and oriented parallel to the epidermis. In acral nevi, melanocytic nests appear at early stage, have well-defined borders, and are uniform in size and shape. As mentioned above, in general, large acral melanocytic lesions with a wide lentiginous intraepidermal component without nest formation is a diagnostic clue of ALM in situ. On the other hand, small acral melanocytic lesions with predominance of symmetrically distributed nests are overwhelmingly nevi. Dendritic morphology is also important in the evaluation of acral lesions. In ALM in situ, the dendrites are thick and numerous as opposed to acral nevus in which they are rarely seen. Another important clue to inspect is the distribution of pigment in the stratum corneum. In acral nevus, the pigment is commonly present in the stratum corneum arranged in columns rather that randomly through the entire width of the lesion (as it is commonly seen in melanoma).
A lesion that needs to be separated from ALM is “MANIAC” nevus (melanocytic acral nevus with intraepidermal ascent of cells) . In these acral nevi, there is a tendency of melanocytes to be scattered throughout the entire thickness of the epidermis, giving the impression of pagetoid spreading, even at the periphery of the lesion. MANIAC nevi are usually seen in younger patients.
Genetics of Acral Lentiginous Melanoma
Recent studies have postulated that DNA repair mechanisms and cell growth pathways are involved in the development of ALM, particularly changes in the MAPK pathways. A recent study assessed the status of the MAP kinase pathways in the pathogenesis of ALM by analyzing the components of the RAS-RAF-MEK-ERK cascades by immunohistochemistry in tissue microarrays [113]. In that study the authors showed that more than half of cases were BRAF positive and most cases were also positive for MEK2, ERK1, and ERK2. RAS was not expressed in most cases and all cases were negative for MEK1. The combination of absence of RAS and expression of MEK2, ERK1, and ERK2 was most seen in invasive cases with high Breslow thickness.
Using comparative genomic hybridization (CGH) , Bastian et al. compared acral melanomas and superficial spreading melanoma, demonstrating that all acral melanomas had at least one gene amplification, significantly more than superficial spreading melanoma [114]. The most frequently amplified regions in acral melanomas occurred at bands 11q13 (47 %) and 22q11–22q13 (40 %). These results suggested that ALM is a distinct type of melanoma characterized by focused gene amplifications occurring early in tumorigenesis. A different study analyzed the frequencies of regional changes in the number of copies of DNA and mutation frequencies in BRAF groups of different melanoma groups (melanomas from the skin with chronic sun-induced damage, melanomas from the skin without such damage, acral melanomas, and mucosal melanomas) [38]. In this study, melanomas arising on the skin with signs of chronic sun-induced damage and skin without sun damage could be correctly classified with 84 % accuracy, and acral melanoma could be distinguished from mucosal melanoma with almost 90 % accuracy. Most melanomas on the skin without chronic sun-induced damage had mutations in BRAF or NRAS, and the majority of melanomas in the other groups had mutations in neither gene. These results suggest that the genetic alterations identified in melanomas at different sites and with different levels of sun exposure indicate that there are distinct genetic pathways in the development of melanoma and implicate CDK4 and CCND1 as independent oncogenes in melanomas without mutations in BRAF or NRAS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


