Melanoma
KEY CONCEPTS
![]() Cutaneous melanoma is an increasingly common malignancy, but it is a cancer that can be cured if detected early. Public education about screening and early detection is one strategy for controlling the increase in incidence and the mortality associated with cutaneous melanoma.
Cutaneous melanoma is an increasingly common malignancy, but it is a cancer that can be cured if detected early. Public education about screening and early detection is one strategy for controlling the increase in incidence and the mortality associated with cutaneous melanoma.
![]() Surgical resection can cure patients with early-stage melanoma.
Surgical resection can cure patients with early-stage melanoma.
![]() The toxicities associated with interferon-α2b (IFN-α2b) therapy are significant and require patient education, close patient monitoring, and appropriate dose modification based on toxicity.
The toxicities associated with interferon-α2b (IFN-α2b) therapy are significant and require patient education, close patient monitoring, and appropriate dose modification based on toxicity.
![]() Patients with locally advanced disease should be evaluated for adjuvant therapy; recommended options include IFN-α2b or participation in a clinical trial.
Patients with locally advanced disease should be evaluated for adjuvant therapy; recommended options include IFN-α2b or participation in a clinical trial.
![]() Metastatic melanoma remains a clinical challenge. At this time, there is not a single standard treatment approach for individuals with metastatic disease. Dacarbazine and temozolomide are considered the most active chemotherapies and can be used as single agents. Combination chemotherapy has not been shown to be superior to single-agent therapy with dacarbazine.
Metastatic melanoma remains a clinical challenge. At this time, there is not a single standard treatment approach for individuals with metastatic disease. Dacarbazine and temozolomide are considered the most active chemotherapies and can be used as single agents. Combination chemotherapy has not been shown to be superior to single-agent therapy with dacarbazine.
![]() As the biology of melanoma has been further delineated, a growing number of potential targets for drug therapy have been identified. BRAF mutations appear in up to 70% of melanoma patients. Vemurafenib is a BRAF inhibitor that has been shown to improve overall survival in patients with this mutation.
As the biology of melanoma has been further delineated, a growing number of potential targets for drug therapy have been identified. BRAF mutations appear in up to 70% of melanoma patients. Vemurafenib is a BRAF inhibitor that has been shown to improve overall survival in patients with this mutation.
![]() Ipilimumab is an option for some individuals with metastatic melanoma. The immune-related toxicities associated with the use of this drug are significant and warrant close patient selection. Individuals require close monitoring and management by an experienced healthcare team. Clinical trials using this drug showed a significant improvement in overall survival.
Ipilimumab is an option for some individuals with metastatic melanoma. The immune-related toxicities associated with the use of this drug are significant and warrant close patient selection. Individuals require close monitoring and management by an experienced healthcare team. Clinical trials using this drug showed a significant improvement in overall survival.
![]() Treatment of melanoma is determined by many factors. As the number of treatment options for patients with metastatic melanoma grows, it will be important to consider disease- and patient-related aspects when determining appropriate therapy.
Treatment of melanoma is determined by many factors. As the number of treatment options for patients with metastatic melanoma grows, it will be important to consider disease- and patient-related aspects when determining appropriate therapy.
INTRODUCTION
Melanoma is the seventh most common cancer in the United States. The incidence of melanoma has steadily increased from the 1970s, and today it is increasing at a faster rate than most other cancer types.1 Although nonmelanoma skin cancers (NMSCs) are the most common malignancies of the skin, cutaneous melanoma accounts for up to 75% of all skin cancer–related deaths. With the rise in the number of melanoma skin cancer and the associated mortality, it is essential to consider issues of care beyond that of disease treatment. Skin cancer prevention and screening have a major impact on public health and on the success of treatment for those individuals diagnosed with both NMSC and melanoma. Skin cancers tend to occur more frequently in older individuals. Therefore, as the population continues to age, effective strategies to prevent, detect, and treat individuals with these cancers are needed.
The incidence of melanoma varies worldwide with the highest rates found in Australia, New Zealand, North America, and Northern Europe. In the United States, about one in every 50 Americans will be diagnosed with melanoma in their lifetime. The lifetime risk is greater in men (2.9%) than women (1.8%) and varies with ethnicity.1,2 The median age at diagnosis is 61 years old.3 In 2013, it was estimated that 76,690 new cases of melanoma would be diagnosed in the United States. Unfortunately, this estimate may not be accurate because many superficial and in situ melanomas are managed in facilities that do not routinely report their cases to cancer registries.4 Childhood and adolescent melanoma is rare with 2% of cases being diagnosed in individuals younger than 20 years old. The incidence in this age group is increasing by 2.9% per year. Young adults between the ages of 15 and 19 years account for about 75% of childhood melanomas. Different than the adult population, the incidence of melanoma appears to be the same between genders except in the 10- to 19-year-old age group in which the incidence is higher in girls than boys.5
The estimated number of individuals expected to die of melanoma in 2013 in the United States is 9,480.4 Survival rates have gradually increased over the past 4 decades. At present, the 5- and 10-year relative survival rates are 91% and 89%, respectively, but survival rates decline with more advanced disease. The overall mortality rate has remained stable.1 Men older than 65 years old have the highest mortality rates from melanoma. Death rates have declined in younger patients. The stabilization of mortality rates appears to be related to efforts at both primary and secondary prevention of melanoma in addition to advances in the treatment and management of melanoma patients.
ETIOLOGY AND EPIDEMIOLOGY
The etiology of melanoma, similar to most other malignancies, is not fully understood. A number of patient-specific factors and environmental factors have been identified (Table 116-1), and it is likely that these factors alone or in combination increase the occurrence of cutaneous melanomas.
TABLE 116-1 Risk Factors for Melanoma
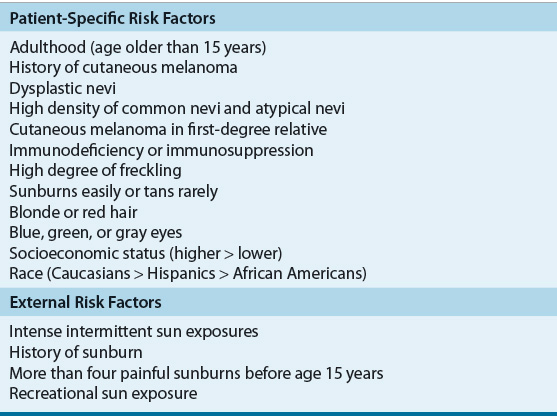
Individual physical characteristics can determine responses to ultraviolet (UV) radiation. Caucasians with fair-colored hair (red or blond), light-colored eyes (blue or green), high degrees of freckling, and those who have a tendency to burn and rarely tan with exposure to sunlight appear especially at risk.5 Clinical and epidemiologic research shows a higher rate of melanoma in those who have extensive or repeated intense sun exposure.5 Intermittent intense sun exposure, blistering sunburns, and the time of life of exposure to the sun now are believed to be critical factors for development of cutaneous melanoma. Individuals with a history of these are at this highest risk. The risk is lower in individuals who had chronic sun exposure without a history of burning and those with occupational exposure. The risk with sunlight and UV radiation seems to be greatest during childhood and adolescence and is more hazardous than exposure during adult life.
One of the most important risk factors for melanoma is the number of melanocytic nevi (pigmented lesions or moles) on the body. The formation of these nevi has been shown to be directly related to cumulative sun exposure. The relative risk of developing melanoma increases with the greater number of typical nevi an individual has. A second risk factor is the presence of atypical melanocytic nevi. Atypical nevi may progress from a normal nevus or be dysplastic from the onset. Up to 20% of melanomas develop from atypical nevi, and individuals with these have an increased risk of developing melanoma compared with the general population. Small congenital melanocytic nevi may be present at birth or within the first few months after birth. About 1% to 3% of newborns are born with pigmented lesions, and the lifetime risk of developing melanoma is related to the size of the nevus.6
Immunocompromised patients are at an increased risk for development of cutaneous melanoma.5,6 Immunodeficiency includes individuals with ataxia telangiectasia, chronic lymphocytic leukemia, Hodgkin lymphoma, and immunosuppression after organ transplant. Acquired immunodeficiency syndrome has been shown to increase the risk of developing cutaneous melanoma and the disease often is more aggressive.7 A personal history of nonmelanoma or melanoma skin cancers is a risk factor for subsequent melanoma and may be associated with a poor prognosis. Xeroderma pigmentosum is a rare skin disorder but does carry an increased risk for melanoma.
A rare but important risk for melanoma is maternal–fetal transfer of melanoma. Although melanoma is not the most common cancer in pregnancy, it is the cancer most likely to metastasize to the placenta and the fetus.6 Maternal–fetal transmission of melanoma is commonly lethal. However, neonates delivered with concomitant placental involvement but without clinical evidence of disease still are considered to be at increased risk for development of disease.
A number of genes have been implicated in melanoma development and progression, and molecular profiling studies have identified several distinct molecular subclasses of melanoma.8 Familial atypical multiple mole syndrome (FAMMS) or dysplastic nevus syndrome is a hereditary disease characterized by a predisposition to develop dysplastic nevi and cutaneous melanoma. About 8% to 10% of cases of melanoma are associated with a family history or hereditary dysplastic nevus syndrome. Patients with FAMMS suggest a risk for melanoma of 400- to 1,000-fold higher than that seen in the general population. The mode of inheritance is somewhat controversial and is believed to be polygenic.
Genetic studies of this heritable trait in families led to the identification of CDKN2A as the familial melanoma gene, located at chromosome 9p21. CDKN2A encodes two distinct proteins: inhibitor of cyclin-dependent kinase 4 (INK4A [inhibitor of cyclin-dependent kinase 4] or p16INK4a) and ARF (alternative reading frame; p14ARF). INK4A regulates cell cycle progression at the G1/S checkpoint by inhibiting the G1 cyclin-dependent kinases that phosphorylate and inactivate the retinoblastoma protein. ARF inhibits p53 degradation; therefore, loss of ARF inactivates p53. The frequencies of CDKN2A mutations vary in melanoma but are found more commonly in individuals with familial inheritance patterns and are associated with multiple cases of melanoma in a family, young age at diagnosis, multiple primary melanomas among family members, and pancreatic cancer.9
One of the major signaling pathways found to be associated with the development of melanoma is the mitogen-activated protein kinase pathway (MAPK), which mediates receptor tyrosine kinases, resulting in activation of RAS and downstream BRAF. Activating BRAF mutations are the most common somatic genetic event in human melanoma, occurring in 25% to 70% of melanoma patients and primarily noted by a single point mutation BRAF (V600E). BRAF does not appear to be an inherited disposition gene, but the high prevalence of BRAF mutations in cutaneous melanoma appears to be an epidemiologic link between UV radiation and melanoma. BRAF mutations are common in melanomas arising from skin with intermittent sun exposure and not as common in melanomas in chronically sun-exposed areas. This may be an early event in the damage to the melanocytes because these mutations are also found in benign and dysplastic nevi.5
Upstream of BRAF, mutations in NRAS and c-Kit have also been found as molecular drivers in the development of melanoma. Mutations in NRAS are found in 15% to 20% of patients. These tumors are associated with more advanced disease at diagnosis, high growth rates, and shorter survival times than those with BRAF mutations.10 c-Kit is a transmembrane receptor tyrosine kinase that when activated signals the MAPK and phosphatidyl-inosital-3-OH kinase (PI3K) pathways, resulting in transcription and cell proliferation. Mutations in c-Kit are commonly found in acral and mucosal melanomas.10
Other genetic alterations involved with the development of melanoma include MITF (microphthalmia-associated transcription factor), a gene that is important to the survival of melanocytes and has been shown to play a key role in melanoma signaling. The melanocortin 1 receptor gene (MC1R), which is associated with the red hair and fair skin phenotype, is involved in melanin synthesis and is more prevalent in individuals with melanoma. NEDD9 modulates metastatic activity and has been found to be unregulated in melanoma. These melanoma-specific pathways give better understanding of the biology of the disease and may lead to better more directed treatment. A variety of other molecular pathways and receptor tyrosine kinases are also being studied to identify their role in the development of melanoma.5
Sunlight is one of the most important environmental factors in the pathogenesis of melanoma. The incidence of melanoma has been associated with latitude and the intensity of solar exposure among susceptible populations. Radiation in the ultraviolet B (UVB) range (280–320 nm) is historically considered to be the critical factor linking sunlight and melanoma, although prolonged exposure to ultraviolet A (UVA) radiation (320–400 nm) also may be important. Use of older UVB-blocking sunscreens may not be as protective as once thought because they allow more sustained sun exposure without any clinical symptoms of burn (e.g., erythema or pain), ultimately resulting in intense irradiation of the skin by UVA light.
PATHOGENESIS
Melanomas most often arise within epidermal melanocytes of the skin, although they can also arise from noncutaneous melanocytes. Human melanocytes are dendritic pigmented cells that arise from the neural crest tissue during early fetal development and migrate over a predictable route to a variety of sites within the body including the skin, uveal tract, meninges, and ectodermal mucosa. In adults, most melanocytes are located at the epidermal–dermal junction of the skin and the choroid of the eye, but they can also be found in other tissues such as the meninges and the alimentary and respiratory tracts. Primary melanoma can arise in any area of the body with melanocytes. The skin is the most frequent site of melanoma; cutaneous melanoma constitutes 90% of all melanomas. Primary melanoma can arise in the eye (ocular melanoma) and less frequently the mucosa and metastatic disease with unknown primary site.5
Normal melanocytes arise from melanoblasts. They undergo a series of differentiation events before reaching a final end-cell differentiation state and can be arrested in their differentiation process at any given state of maturation without loss of their proliferation capacity. Melanocytes adhere to the basement membrane of the epidermis and, despite a resting state, maintain a lifelong proliferation potential. The existence of melanoma stem cells has been suggested from work with melanoma cell lines.8
Melanocytes synthesize melanin to protect various tissues, such as the skin, from UV radiation–induced damage and reach the keratinocytes in the upper layers of the epidermis via dendrites. Tyrosinase is an essential enzyme within melanosomes that synthesizes melanin. They are resistant to severe UV radiation, unlike keratinocytes, and their survival leads to the proliferation of mutated genes.5
Skin melanocytes transform from preexisting nevocellular nevi in the development of melanoma. A series of distinct steps are involved in the development and progression of melanoma from melanocytes. The pathologic components of the progression in human melanoma involve a series of morphologic stages: melanocytic atypia, atypical melanocytic hyperplasia, radial growth phase in which limited growth and radial expansion of the nevi may occur without metastatic competence, primary melanoma in the vertical growth phase with or without in-transit metastases, regional lymph node metastatic melanoma, and distant metastatic melanoma.5 Primary melanoma is characterized by radial growth and limited vertical thickness (<0.75 mm). Primary melanoma demonstrates little tendency to metastasize. Melanoma has a potential for metastasis formation with the onset of a vertical growth phase. Therefore, the thickness of a primary melanoma is an important prognostic factor and is used in the staging classification of cutaneous melanoma. Of note, melanomas can skip steps in this development pathway.
Normal melanocytes require growth factors for proliferation, but melanoma cells can proliferate without growth factors. Melanoma cells secrete a variety of growth autocrine and paracrine factors that may facilitate proliferation. Additionally, with disease progression, melanoma cells increase production of certain growth factors and cytokines. The PI3K–AKT pathway often is overactive in melanoma. Integrins and growth factors promote growth and survival of melanoma through these pathways.
Basic fibroblast growth factors (bFGFs) are thought to be important mediators of growth stimulation and cell survival, act as motility factors for melanoma cells, and upregulate serine proteinases and metalloproteinases. Melanoma cells are strong producers of chemoattractive proteins such as interleukin-8. Vascular endothelial growth factor (VEGF) can be triggered in the vertical growth phase.11 Most of these changes occur between the radial growth phase and vertical growth phase of primary melanoma, and metastatic cells often show the highest cytokine production.
Understanding the biology of melanoma has provided potential targets for drug therapy.12 For example, the role of bFGF in the pathogenesis of melanoma has led to investigation of antisense oligonucleotides to block bFGF. Other pathways, such as MAPK pathway, have been targeted by RAF and MEK inhibitors and the PI3K/AKT pathway by mTOR (mammalian target of rapamycin) inhibitors. As pathways are identified and as agents that inhibit these pathways enter clinical trials and practice, there is growing excitement about the opportunities to impact treatment of melanoma in new and effective ways.
Immune factors appear to be involved in the progression of melanoma more often than in most other solid tumors.5 Spontaneous cancer regressions are rare but are a well-documented phenomenon seen in melanoma. Focal regression in primary melanoma has been reported. Tumor regression appears to be associated with host immunity.
A number of different tumor antigens have been identified in the cellular membrane and cytoplasm of melanoma cells and are referred to as melanoma-associated antigens. Ganglioside antigens have been of particular interest in the development of immunotherapy for melanoma. A large number of monoclonal antibodies to melanoma-associated antigens have been developed and are being evaluated in clinical trials for diagnosis of and therapy for melanoma.
The humoral and cellular responses of individuals with melanoma that express melanoma-associated antigens have been described and provide the rationale for immunotherapy in the management of metastatic melanoma.5 Melanoma-directed antibodies have been isolated in the sera of patients with melanoma. The presence of antimelanoma antibodies in the sera of patients correlates with the clinical status of the patients, and the antibodies gradually disappear from the serum as the disease progresses. This phenomenon may be explained by the possible formation of anti-idiotype antibodies directed against the antimelanoma antibodies, an increase in the circulation of soluble tumor antigens that saturate all antibody combining sites, increased levels of immunosuppression, or absorption of antibodies on the tumor mass.
Interest has focused on the role of cell-mediated immune response in melanoma. Specific cell-mediated responses may play a role in tumor regression, but the role of specific cells, such as cytotoxic T lymphocytes (CTLs), is not fully understood. Tumor-infiltrating lymphocytes (TILs) have been shown in vivo and in vitro to possess antitumor reactivity. TILs contain a large number of mature tumor-specific lymphocytes and have been a target for manipulation in immunotherapeutic approaches for melanoma.5 Two identified targets are cytotoxic T lymphocyte antigen 4 (CTLA-4) and toll-like receptor 9 (TLR9). CTLA-4 is a glycoprotein expressed on the surface of activated T cells that appears to have an inhibitory effect on T cells. Blocking the effect of CTLA-4 is an effective strategy for increasing the T-cell antitumor response.
HISTOLOGIC SUBTYPES
Cutaneous melanomas are categorized by growth patterns. Four major histologic subtypes or growth patterns of primary cutaneous melanoma have been identified: superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, and acral lentiginous melanoma. Clinical outcomes of the four major melanoma subtypes are similar if the comparison controls for depth of penetration or tumor thickness. Any of the four subtypes can present as an amelanotic variant. Amelanotic melanomas appear to be devoid of clinically apparent pigmentation. Two less common types of melanoma include desmoplastic melanoma and lentiginous melanoma. Desmoplastic melanoma is more commonly seen in older individuals, and its clinical presentation is similar to that seen in NMSCs. If a biopsy of the lesion is not obtained, the disease may be mismanaged. Lentiginous melanoma is histologically different than the four major subtypes. Uveal melanoma is considered a separate disease from cutaneous melanoma.
Superficial spreading melanoma is the most common morphologic type of cutaneous melanoma, accounting for about 70% of all melanomas.5 The lesions usually arise from a preexisting nevus, known as a precursor lesion, and evolve slowly over 1 to 5 years. At some point, superficial spreading melanoma may progress to a more rapid growth phase. Early in lesion development, the superficial spreading melanoma is flat, but the surface becomes irregular and asymmetrical as the lesion progresses. The lesion enlarges when it enters into a rapid growth phase, and the edges appear notched or lacy. The lesions can be blue, black, or pink. Areas within the lesion may be hypopigmented. These patches of color variation, specifically the hypopigmented areas, are thought to be associated with tumor regression within the lesion or pigment inconsistency. The clinical differential diagnosis of superficial spreading melanoma includes both benign and malignant skin disease. This subtype is sometimes confused with seborrheic keratoses or pigmented basal cell carcinoma. Superficial spreading melanomas may occur at any anatomic site on the body, but they are more commonly seen on the back in men and on the legs in women. This subtype of melanoma is more common in women. The mean age of diagnosis of superficial spreading melanoma is 51 years, which is earlier than that seen for other subtypes. Superficial spreading melanoma usually occurs after puberty.
Lentigo maligna melanoma represents 10% to 20% of melanomas and is commonly found on the head and neck. It is unique from other histologic subtypes; because of its prolonged radial growth phase, it does not have the same propensity to metastasize.5 Lentigo maligna melanoma arises on chronically sun-exposed sites in older individuals and presents as a freckle-like lesion. Lentigo maligna melanomas are generally large (>3 cm), flat, and tan-colored lesions with shades of brown and black. The lesions gradually grow and develop darker, with asymmetric flecks in areas. Lentigo maligna melanoma is uncommon before age 50 years and may have been present for more than 5 years. Only about 5% to 8% of lentigo maligna melanomas evolve into invasive melanoma, which is characterized by nodular development within the flat precursor lesion. Lentigo maligna melanoma can be difficult to distinguish from solar lentigo, which typically is a smaller and evenly pigmented flat-appearing lesion.
Nodular melanoma is the second most common growth pattern of melanoma, occurring in 15% to 30% of patients. Nodular melanoma is a pure vertical growth phase disease. In nodular melanoma, a small, expansive nodule in the papillary dermis invades the reticular dermis and subcutis. The radial growth phase is absent at all times. Nodular melanomas are more aggressive and develop more rapidly than superficial spreading melanoma. Nodular melanomas are dark blue–black and often uniform in color with a shiny surface, although a small percentage of nodular melanomas are amelanotic and have a fleshy appearance. Nodular melanomas are raised and often symmetric. They can occur at any age, typically around 50 years of age, and are most common on the trunk, head, and neck. Nodular melanomas are more common in men. Of note, nodular melanomas can resemble traumatized nevi.
Acral lentiginous melanoma makes up about 5% of melanomas and is most likely not related to UV exposure. It presents as three distinct clinical subtypes: melanoma on the palms of the hands or soles of the feet, subungual melanoma, and mucosal melanoma.6 Most acral lentiginous melanomas are located on the soles of the feet and look like a large tan or brown stain. The lesions often have irregular convoluted borders. The initial macular component of palmar/plantar melanomas can be masked by the thickened stratum corneum at these sites. Many of these lesions look verrucous in appearance making them difficult to distinguish from warts by the untrained eye. Suspicious lesions on the palms or soles of the feet should be evaluated. Acral lentiginous melanoma includes subungual melanoma, which arises in the nail matrix or nail bed. The most common presentation is a brown or black line in the great toe or the thumbnail. Mucosal melanoma is rare but can occur on any mucosal surface. Mucosal melanoma occurs most commonly in the oropharyngeal mucosa followed by the anal and rectal mucosa, genital mucosa, and urinary mucosa. Unfortunately, mucosal melanoma often does not become clinically apparent until the mass is large or the lesion bleeds. Acral lentiginous melanoma occurs in fewer than 10% of Caucasians with melanoma but is the most common type of melanoma reported in individuals with a dark complexion (e.g., African Americans, Asians, and Hispanics). Similar to lentigo maligna melanomas, this subtype is characterized by a protracted radial growth phase.
Uveal melanoma is the most common primary intraocular malignancy seen in adults but is an uncommon tumor.13 Unlike cutaneous melanoma, the frequency and mortality rates of uveal melanoma have remained steady. This melanoma arises from the pigmented epithelium of the choroid. Iris melanoma is a subset of uveal melanoma and tends to have a more benign course. The risk of metastasis varies with the histologic type and size of the tumor as well as the location in the eye. Metastases occur most frequently in the liver but have been documented in a variety of tissues.
The ability to predict the metastatic potential of melanomas would be a valuable prognostic tool. An attempt to predict the likelihood for metastasis is based on radial and vertical growth phases. Radial growth phase describes the early stage of melanoma when the tumor is thin and primarily intraepidermal in location. By definition, malignant melanoma in situ is a form of radial growth phase melanoma. Vertical growth phase is the stage of melanoma with clear metastatic potential.
CLINICAL PRESENTATION
Benign nevi often occur in sun-exposed areas and typically are 4 to 6 mm in diameter (about the size of a pencil eraser), raised or flat, uniform in color and round in shape. Dysplastic nevi are believed to be a link between benign nevi and melanoma. Dysplastic nevi tend to be larger than common nevi (>5 mm), appear as flat macules with asymmetry, have a fuzzy or ill-defined shape, and vary in color. Compared with melanoma lesions, dysplastic nevi appear less evolved.
The initial clinical presentation of melanoma often is a cutaneous lesion and depends on the histologic subtype and the stage of development of the lesion. The lesion can be located anywhere on the body but is most commonly discovered on the lower extremities in women and on the back and trunk in men. The cardinal clinical feature of a cutaneous melanoma is a pigmented skin lesion that changes over a period of time. Any changes in the skin surrounding a nevus, including redness or swelling, are important clinical signs. Uncommonly, the sensation of the lesion may become itchy or tender and painful. Friability of the lesion, resulting in bleeding or oozing, is a danger sign. Perhaps the most important warning sign of danger is the evolution in any characteristic of a lesion. A biopsy of the lesion is critical to establish diagnosis of melanoma. Subsequent pathologic interpretation of the biopsy will help provide information on prognosis and treatment options. An excisional biopsy with a 1- to 2-mm margin of normal-appearing skin is recommended for a suspicious lesion and should include a portion of underlying subcutaneous fat for microstaging. For larger lesions with which an excisional biopsy is impractical, an incisional or punch biopsy can be performed and should include a core of full-thickness skin and subcutaneous tissue. When excisional biopsies are not appropriate, as with the face or palmar surface of the hands, a full-thickness incisional or punch biopsy is preferred. A shave biopsy is never appropriate because it can underestimate the thickness of the lesion and may not fully remove the lesion. Additionally scarring may mask the remaining tumor.
CLINICAL PRESENTATION
Evaluation of any individual with a suspected melanoma includes a complete history and total-body skin examination. The focus of the patient history is identifying potential risk factors. Risk-related questions include an assessment of family history of melanoma, personal history of skin cancer or nevus excisions, sun exposure, and phenotype. Total dermatologic examination is necessary to determine melanoma risk factors (e.g., mole pattern, mole type, or freckling) and for staging. Melanoma commonly spreads to the lymph nodes; therefore, individuals suspicious for advanced disease should have their lymph nodes examined for lymphadenopathy. Lactate dehydrogenase (LDH) should be measured because elevated serum levels have been shown to be an independent predictor of decreased survival.14 In addition, any other signs or symptoms suggestive of metastatic disease should be completely evaluated.
The diagnosis of melanoma is complicated by the number of pigmented moles (melanocytic nevi) and nonmelanocytic lesions that resemble melanoma. An average of 10 to 40 ordinary nevi can be found on the skin of white adults. Nonmelanocytic pigmented lesions, such as seborrheic keratoses, pigmented basal cell carcinoma, and vascular lesions, also can appear similar to a melanoma lesion. In childhood melanoma, commonly the lesions are thicker at the time of diagnosis. This may be in part due to the low level of suspicion by pediatricians, the fact that many melanomas associated with congenital nevi develop in the dermis rather than the epidermis, and histologic uncertainty.6
![]() Improved survival rates for melanoma have been attributed to the identification and treatment of disease at an early stage when the disease is limited and has not yet metastasized. It follows that one strategy to improve survival rates would be to increase efforts to identify early-stage melanoma. The cost effectiveness of massive screening for all adults by a physician has never been demonstrated. However, routine examination of the skin by physicians is recommended for individuals, adults, and children who are at high risk. The entire cutaneous surface, including the scalp, should be examined.
Improved survival rates for melanoma have been attributed to the identification and treatment of disease at an early stage when the disease is limited and has not yet metastasized. It follows that one strategy to improve survival rates would be to increase efforts to identify early-stage melanoma. The cost effectiveness of massive screening for all adults by a physician has never been demonstrated. However, routine examination of the skin by physicians is recommended for individuals, adults, and children who are at high risk. The entire cutaneous surface, including the scalp, should be examined.
It has been estimated that about 50% of the initial melanoma lesions found are discovered by self-examination. Therefore, one of the most direct strategies to improve early detection would be a method to increase effective skin self-examination (SSE) by the individual, the individual’s partner, or a caregiver. Identification of early melanoma allows the opportunity to treat the lesions when they are thin and curable. Persons who perform SSE present for care at an earlier stage in the disease process and have 50% less advanced melanoma and lower mortality rate from the disease.5 Healthcare individuals who routinely work with the public, such as community pharmacists, have an opportunity to increase public awareness concerning the benefits and appropriate methods for SSE. Educational pamphlets describing SSE (Table 116-2) for the public are widely available through the American Cancer Society, American Academy of Dermatology, and Skin Cancer Foundation. If a newly discovered pigmented lesion is identified or if a preexisting pigmented lesion changes, the individual should be evaluated by a physician immediately.
TABLE 116-2 Self-Examination of Suspicious Moles

Skin self-examination is of special interest in elderly adults. As the population of older adults (≥65 years of age) increases, it is expected that the mortality rate from melanoma also will increase. Barriers to successful SSE in elderly adults, such as failing eyesight, lack of partners, and poor memory, impact older adults in detecting new or changing lesions. These barriers, coupled with the higher incidence of melanoma in men, present challenges and opportunities for healthcare professionals to target education on this growing segment of our population.
STAGING AND PROGNOSTIC FACTORS
The size of a primary melanoma lesion is associated with the likelihood of metastases. As such, Breslow tumor thickness of the primary melanoma lesion is commonly used as prognostic factor to determine predicated outcomes.15 Tumor thickness is quantified to the nearest tenth of a millimeter with an ocular micrometer, measuring from the top of the granular layer of the overlying epidermis to the deepest contiguous invasive melanoma cell. The correlation between tumor thickness and probability of tumor metastases is strong but does not include aspects such as tumor satellites, defined rather arbitrarily as skin involvement within 2 cm of the primary lesion, and vascular invasion. It was once thought that the presence of satellite nodule(s) had the same impact on prognosis as a high-risk primary lesion (tumor thickness >4 mm). It is now known that patients with satellitosis have a worse prognosis than patients with thick primary lesions, and prognosis is more similar to that of patients with nodal metastases. Mitotic rate has now emerged as another important prognostic factor for developing metastatic disease. Mitotic rate is defined as the number of mitosis per square millimeters. Increasing mitotic rate represents a more aggressive lesion and is associated with a poorer survival rate despite tumor size. Ten-year survival rates drop by 8% for a nonulcerated T1 melanoma with a mitotic rate of less than 1/mm2 compared with a lesion with a mitotic rate of greater than 1/mm2.16 The American Joint Committee on Cancer (AJCC) developed a staging system for melanoma that divides patients with localized melanoma into four stages according to microstaging criteria of Breslow.17 In addition to consideration of the primary lesion, the AJCC staging system includes aspects of the tumor satellite, extent of lymph node involvement, and presence of metastatic disease.17 Analysis of several large databases worldwide identified areas in which the AJCC staging system, which was published in 1997, did not reflect the natural history of melanoma. Issues such as the appropriate cutoff values for primary tumor thickness, ulceration of the melanoma, and satellite lesions of the primary tumor should be considered when making decisions about therapy.17 The cutoff values initially proposed by Breslow for primary tumor thickness were initially used in the AJCC staging system, but it appears that cutoff depths of 1, 2, and 4 mm of thickness may better predict overall survival. Melanoma ulceration and increased mitotic rate within a primary melanoma are both associated with decreased survival and thus are the most considerable prognostic factors in patients with localized disease.14 The revised AJCC staging system for cutaneous melanoma was published in 2002 and updated in 2009.14,17 It is important to carefully examine older clinical trials to determine which staging system was used to determine patient inclusion and exclusion criteria, as results may differ based on these patient criteria. Revisions of the new melanoma staging system include (a) principal prognostic factors for localized disease includes melanoma thickness, ulceration, and mitotic rate; (b) mitotic rate replaces invasion as primary criterion for T1b tumors ; (c) number of metastatic nodes, tumor burden, and ulceration define the nodes (N) category for patients with regional metastasis; (d) presence of microscopic nodal metastasis classifies a patient as stage III; and (e) two dominant components are site of distant metastases and presence of elevated serum LDH for metastatic disease.14 Clinical staging includes microstaging of the primary melanoma and clinical and radiologic evaluation. It is used after complete excision of the primary melanoma with clinical assessment for regional and distant metastasis. Pathologic staging includes microstaging of the primary melanoma and pathologic information about the regional nodes after partial or complete lymphadenectomy. At this time, it appears that patients with very limited disease (in situ or stage 0) do not require pathologic evaluation of lymph nodes (Tables 116-3 and 116-4). As with other solid tumors, the presence of regional lymph node involvement is a powerful predictor of tumor burden and patient outcome. In the past, the primary method for determining nodal status was surgical resection and analysis of the lymph nodes via a regional lymph node dissection. The extent of lymph node dissection was determined by the anatomy of the area of the lesion. In recent years, preoperative lymphoscintigraphy and intraoperative sentinel node mapping have become more widely used methods for identifying the first or sentinel lymph node in the direct pathway of lymph drainage from the primary cutaneous melanoma. Sentinel lymph node biopsy (SLNB) is a minimally invasive procedure that determines if a patient is a candidate for a complete lymph node dissection. The rationale for lymphatic mapping and subsequent SLNB is based on the observation that regions of the skin have patterns of lymphatic drainage to specific lymph nodes in the regional lymphatic basin. The sentinel lymph node is believed to be the first node in the lymphatic basin into which the primary melanoma drains. Unlike other solid tumors, melanoma appears to progress in an orderly nodal distribution. Evaluation of sentinel nodes has been used for detection of micrometastases in breast cancer and in melanoma. SLNB allows for more thorough examination of a single sentinel node than is possible when examining multiple lymph nodes with a lymph node dissection and may be most useful for melanomas located in ambiguous drainage sites such as the head and neck areas. SLNB is associated with low false-negative rates and low complication rates.18 Detection of clinically undetectable disease in a lymph node basin that is not directly adjacent to the primary lesion may allow for upstaging of patients who initially are believed to have node-negative disease. The American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guidelines now recommend SLNB for patient with any intermediate-thickness melanoma.19
TABLE 116-3 Melanoma Tumor, Node, Metastasis Classification
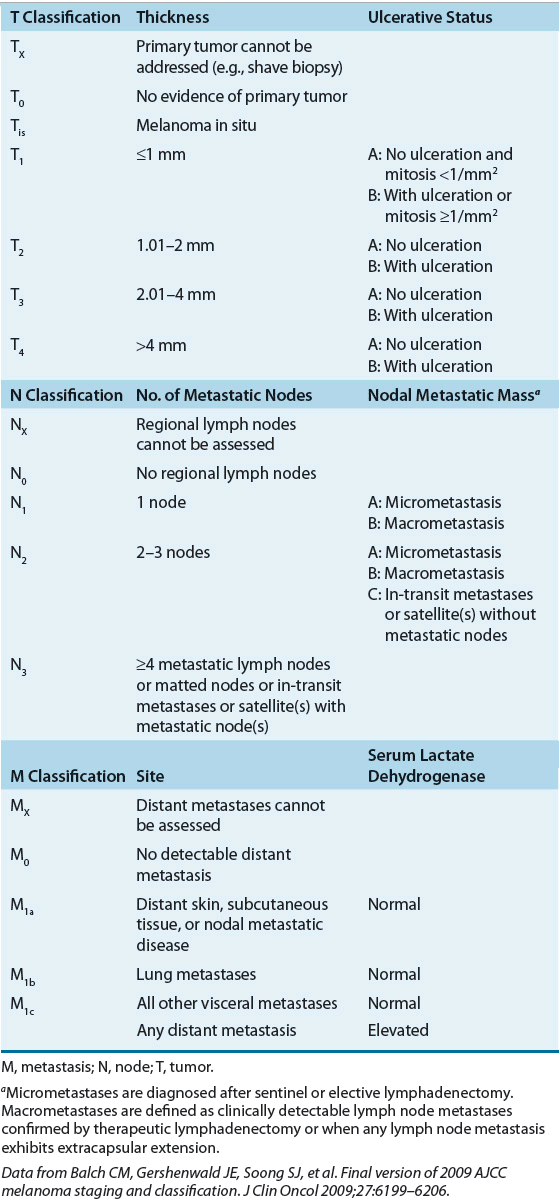
TABLE 116-4 American Joint Committee on Cancer Tumor (T), Node (N), Metastasis (M) Stage Grouping for Cutaneous Melanoma
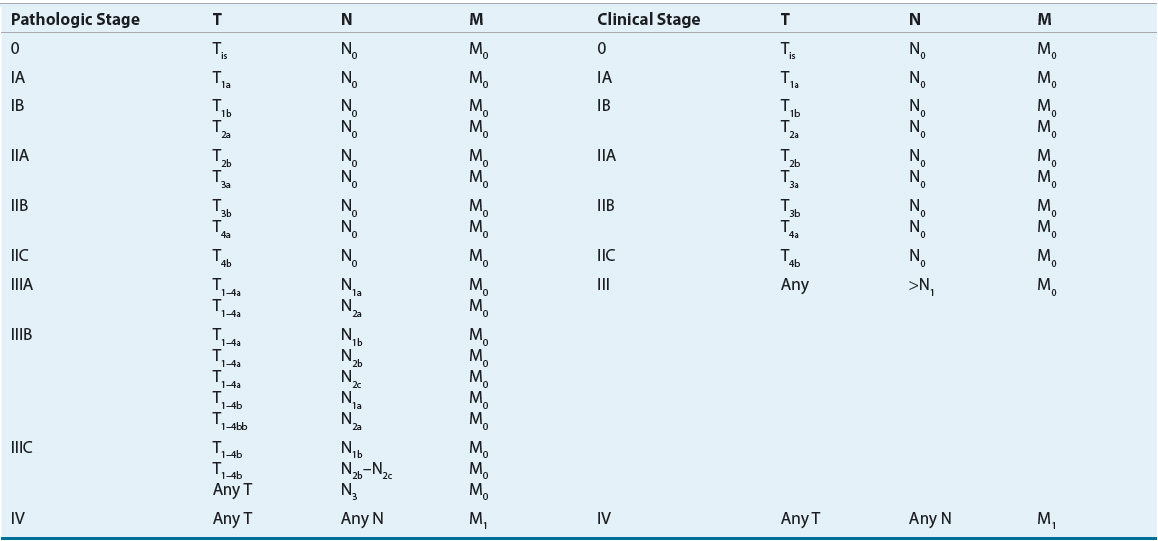
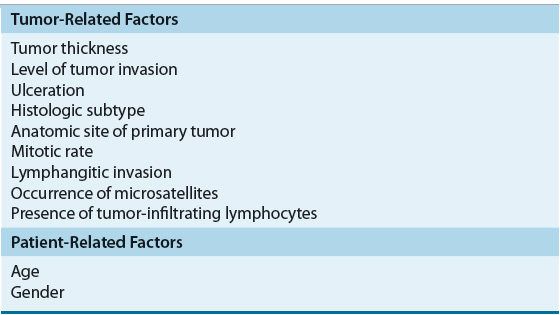
The stage of melanoma at the time of diagnosis is one of the primary indicators of the natural history of the disease and contributes to prognosis. Tumor thickness, level of tumor invasion, and ulceration all contribute to the stage of a patient and the overall outcome. Other factors such as tumor growth pattern or histological subtype, mitotic rate, density of TILs infiltrating the tumor tissue, elevated LDH level, satellite lesions, angiolymphatic invasion, gender, and age also have been reported to influence survival (Table 116-5). The location of the primary tumor on the skin is also important because individuals with tumors of the extremities have an increased survival compared with those with axial, neck, head, and trunk tumors. In addition, a number of additional prognostic factors have been identified in patients with advanced disease. The number of metastatic sites, disease involvement of the gastrointestinal tract, liver, pleura, or lung, Eastern Cooperative Oncology Group (ECOG) performance status of 1 or greater, male sex, and prior immunotherapy have been associated with poor prognosis.20
TABLE 116-5 Prognostic Factors for Cutaneous Melanoma
TREATMENT
Desired Outcomes
Treatment of cutaneous melanoma depends on the stage of disease. Local disease is managed and often cured with surgical ablation. Regional disease is treated with surgical resection of the primary lesion and, depending on the risk of recurrence, possibly adjuvant therapy in an effort to eradicate any residual disease and cure the patient. The use of adjuvant therapy after surgical resection and the role of interferon-α (IFN-α) as adjuvant therapy remain controversial. When disease becomes metastatic, the treatment goals are to slow tumor progression, prolong life, and improve quality of life. The approvals of ipilumumab and vemurafenib have dramatically changed the management of metastatic melanoma. Both agents are more efficacious than standard chemotherapy treatment options, dacarbazine and temozolomide. Numerous clinical trials have evaluated single-agent and combination chemotherapy, immunotherapy, targeted therapy, and biochemotherapy regimens. Patients with advanced disease should have their tumors tested to document mutational status in an effort to direct appropriate therapy.
Surgery
![]() Patients who present with a suspicious pigmented lesion should undergo a full-thickness excisional biopsy, if possible. Sites for which excisional biopsy is inappropriate include the face, palm of the hand, sole of the foot, distal digit, and subungual lesions. A full-thickness incisional or punch biopsy is preferred in these cases to provide microstaging and ultimately to determine therapy.
Patients who present with a suspicious pigmented lesion should undergo a full-thickness excisional biopsy, if possible. Sites for which excisional biopsy is inappropriate include the face, palm of the hand, sole of the foot, distal digit, and subungual lesions. A full-thickness incisional or punch biopsy is preferred in these cases to provide microstaging and ultimately to determine therapy.
Localized cutaneous melanoma can often be cured with surgical excision. The cure rates for melanomas smaller than 1 mm are as high as 98%.5 The extent of the excision margin is important in preventing local recurrence and ultimate survival. For melanoma in situ, excision of the visible lesion or biopsy site with a 0.5 to 1 cm border of clinically normal skin and a layer of subcutaneous tissue with confirmation of histologically negative peripheral margins is recommended. The recommended clinical margin for invasive melanoma depends on the tumor thickness. Excision with a 1 cm margin of clinically normal skin and underlying subcutaneous tissue is recommended for invasive melanomas 1 mm or smaller thick.20,21 The appropriate margin of excision for melanomas between 1 and 2 mm in thickness is controversial. A study suggests the risk of locoregional recurrence is higher when melanomas that are at least 2 mm thick are excised with a 1-cm margin rather than a 2 cm margin.22 Current National Comprehensive Cancer Network (NCCN) guidelines recommend a 1 to 2 cm margin for melanoma with tumor thickness of 1.01 to 2 mm.20 Lesions that are 2 to 4 mm thick should be excised with a 2 cm margin. Primary tumors more than 4 mm thick require at least a 2 cm margin, but whether a larger margin is beneficial is not clear. Surgical management of lentigo maligna melanoma is problematic because subclinical extension of atypical junctional melanocytic hyperplasia may extend beyond the visible margins. Complete excision of these lesions is important.
When isolated regional lymph nodes are detected via physical examination in the absence of distant disease, therapeutic lymphadenectomy is recommended. The extent of therapeutic lymph node dissection often is modified according to the anatomic area of the lymphadenopathy. Prophylactic lymphadenectomy in all patients is not recommended. Although a subgroup of patients with early-stage melanoma will have microscopic metastatic disease in nonpalpable lymph nodes, prophylactic regional lymph node dissection does not prolong survival or decrease time to relapse in randomized clinical trials.5,23 Selective regional lymphadenectomy performed after scintigraphic and dye lymphographic identification of the affected sentinel draining lymph node(s) is the standard of care for melanomas more than 1 mm thick. If the sentinel node is found to have micrometastatic melanoma, regional dissection of the involved nodal basin is performed. If the lesion is 0.75 to 1 mm in thickness with ulceration or is Clark level IV or V, lymphatic mapping with SLNB may be considered based on patient characteristics such as ulceration of the tumor.24 Of note, the likelihood of detecting metastatic disease in the sentinel lymph node depends on tumor thickness. The likelihood of detecting metastatic disease is about 1% in tumors that are smaller than 0.8 mm but increases to more than 30% in tumors 4 mm thick.24 The Multicenter Selective Lymphadenectomy Trial II is currently enrolling patients to assess whether or not a complete lymph node dissection after a positive SLNB improves overall survival. SLNB results are important for accurate staging, for therapeutic lymphadenectomy, and to aid in the decision to offer adjuvant treatment.18,23
One of the most important aspects of surgical management of cutaneous melanoma is the role of patient follow-up.20 Postsurgical follow-up of patients who have had a melanoma excised is essential to monitor for undetected metastatic disease and the development of a second primary cutaneous melanoma or nonmelanoma primary malignancy. Scheduled screening in addition to routine surgical follow-up is required for any patient with a melanoma; the recommended frequency and duration depend on the stage of melanoma. The optimal duration of follow-up remains controversial. Most patients who develop recurrent disease do so in the first 5 years after treatment, but late recurrences more than 10 years after surgery have been observed. The increased lifetime risk of developing a second primary melanoma supports lifetime dermatologic surveillance for all patients.
Curative surgery usually is limited to patients with early-stage disease. A patient with stage III melanoma commonly has lymph node involvement, but in-transit metastases also may occur. In-transit metastasis is the clinical manifestation of tumor that develops in lymphatics between the primary melanoma and the regional lymph node basin.5 In-transit metastases are more than 2 cm from the original lesion. In-transit metastases are more common in individuals with thick, ulcerated lesion. Surgery is used for management of in-transit lesions, and the goal is complete resection. Unfortunately, subsequent recurrence in the same extremity often occurs after initial resection of in-transit metastases.
The role of surgery beyond that of cure is less clear, although surgery may offer palliation for patients with isolated metastases.23 Resection of isolated lesions in the brain and lungs may be appropriate in certain cases and should be evaluated based on individual patient criteria. Surgery can be an option when the lesion is accessible and when the lesion may cause problems if not removed. Surgery can extend survival time in select patients with metastatic disease. Patients whose metastases can be completely resected may experience improved quality of life, improved overall survival, and occasionally long-term disease control.23
Brain metastasis is a frequent complication of advanced melanoma. About 20% to 50% of patients with stage IV disease develop clinically apparent central nervous system (CNS) involvement. Surgical resection, with or without radiation, has been used in select individuals. More recently, high control rates of brain metastases have been achieved with focal radiation therapy such as linear accelerator–based stereotactic radiosurgery or gamma-knife technologies.25 Melanoma in the gastrointestinal tract can lead to bowel obstruction, and appropriate resection or bypass may provide significant relief of symptoms. Despite the lack of controlled clinical trials, the impact on palliative surgery should be evaluated in the context of a patient’s comfort and quality of life. Surgery may be an appropriate option if the perceived outcome is to provide patient comfort. On the other hand, surgery may constitute a significant physical challenge or financial burden to a patient with a limited life expectancy. The clinical scenarios involving surgical resection should be fully evaluated in terms of overall quality of life.
The risk of relapse and death after resection of a local or regional cutaneous melanoma is the primary determinant for use of adjuvant therapy after primary resection. Adjuvant trials have focused on patients at intermediate or high risk for recurrence.
Immunotherapy
Melanoma is considered one of the most immunogenic solid tumors, and it appears to interact with and respond to the immune system of the host in which it arises. Spontaneous regressions of melanoma suggest the importance of the immune system in disease modulation. Lymphoid infiltration into the primary melanoma also suggests that immunomodulation may impact the biology of melanoma. Early work showed that nonspecific immunomodulators, such as levamisole and bacillus Calmette-Guérin (BCG), for treatment of melanoma were associated with some regression of the tumor, although many of these responses were limited and short-lived. Because melanoma is generally resistant to traditional treatment modalities such as radiation and chemotherapy, immunotherapy offers an avenue of treatment. Although the complete response rate seen in patients with melanoma treated with biotherapy is relatively low, the durability of responses in individuals who respond can be significant. Remaining unanswered questions include what is the best approach to biotherapy in a patient with melanoma and can biotherapy be combined with other available and emerging antineoplastic therapy.
Interferon
One of the oldest, and most controversial, immunotherapy approach-es for the treatment of melanoma is the use of IFNs. The IFNs are a group of proteins with diverse immunomodulatory and antiangiogenic properties. A number of studies have evaluated various doses and schedules of recombinant IFN for treatment of metastatic melanoma. Response rates in metastatic melanoma range from 10% to 30%, and overall response rates are about 15% for IFN-α. Unfortunately, the optimal dose, treatment schedule, and treatment combinations and regimens have not been established for management of metastatic melanoma.5
In clinical trials of IFN therapy for patients with metastatic melanoma, response rates were highest in patients with minimal disease. Responses were seen at all sites of disease but were most frequent in subcutaneous, lymph node, and pulmonary metastases. The success of IFN in patients with minimal disease encouraged investigators to evaluate the role of adjuvant IFN after curative surgical resection in patients who were at high risk for recurrent disease (bulky disease or regional lymph node involvement). Early trials of short-term or low-dose regimens of IFN-α did not demonstrate a survival benefit in the adjuvant setting. In an attempt to optimize response in the adjuvant setting, maximum tolerated doses of IFN-α were administered for 1 month followed by prolonged therapy of IFN-α at more tolerable doses for 48 weeks. The rationale for the intensive induction phase was to provide peak IFN levels sufficient to inhibit tumor growth and avoid the development of anti-IFN antibodies. A large, multicenter cooperative group trial (E1684) of adjuvant IFN-α2b versus observation was designed for 287 patients with high-risk (stages IIB and III disease based on the 1997 AJCC staging criteria) melanoma after curative surgical resection. IFN-α2b was given IV as an induction therapy at maximum tolerated doses of 20 million IU/m2 per dose 5 days per week for 4 weeks in an outpatient setting; treatment was continued for 48 weeks with subcutaneous IFN-α2b 10 million IU/m2 per dose 3 times per week at home. This therapy now is often referred to as high-dose interferon (HDI). With a median followup period of 6.9 years, patients treated with HDI had significantly longer relapse-free and overall survival compared with patients who were observed after surgical resection (1.72 vs. 0.98 years and 3.8 vs. 2.8 years, respectively).26 Both the 5-year relapse-free and overall survival rates were higher with HDI. With longer follow-up (median, 12.6 years), however, the difference in overall survival was no longer significant.27 Further analysis showed that the greatest reduction in melanoma recurrence occurred during the first few months of treatment. Subgroup analysis of this study indicated that patients with large primary tumors and node-negative disease (T4N0M0) did not receive the same benefit from therapy, but the small number of patients in this group made it difficult to draw definite conclusions about the role of IFN for adjuvant therapy in this setting.
Pegylated IFN-α2b has also been evaluated in the adjuvant setting. The European Organization for Research and Treatment of Cancer (EORTC) 18991 trial evaluated 1,256 patients with resected stage III melanoma. Patients were randomized to observation or pegylated IFN. Pegylated IFN was given less frequently (once weekly) compared with nonpegylated IFN. Updated results demonstrated an improvement in relapse-free survival but no difference in overall survival or distant metastasis-free survival.28 Based on these data, the Food and Drug Administration (FDA) approved pegylated IFN-α2b (Sylatron) as an option for adjuvant treatment.
High-dose interferon treatment is associated with multiple toxicities, including flulike syndrome. Other toxicities include depression, nausea, weight loss, fatigue, myelosuppression, elevations in liver function tests, and renal insufficiency. Toxicities of IFN therapy in the adjuvant HDI trials were common and severe, and most patients required dose reductions or delays at some point during treatment. Dose modifications were required for dose-limiting constitutional symptoms, myelosuppression, and hepatic toxicities. Approximately three-quarters of patients were able to complete the year of therapy in an outpatient setting.
One of the strategies for reducing the toxicities associated with IFN was to modify the dose and duration. A subsequent ECOG trial (E1690) of low-dose IFN (LDI; 3 million IU per dose given subcutaneously three times weekly) for 24 months compared with the HDI regimen described earlier versus observation did not demonstrate an overall survival advantage of HDI versus observation.29 At a median followup period of 52 months, the 5-year estimated relapse-free survival rates for HDI, LDI, and observation were 44%, 40%, and 35%, respectively. Relapse-free survival was significantly longer in the HDI group, prolonging the median time to relapse by 10 months compared with observation and LDI. With longer follow-up, however, the difference in relapse-free survival was no longer significant.29 A significant overall survival benefit was not seen for HDI or LDI compared with observation, although the investigators speculated that this analysis of survival was affected by the number of patients in the observation arm who received IFN therapy after disease progression.29
The use of IFN in the adjuvant setting remains controversial. Although the HDI regimen is used in the United States, the LDI strategy remains standard in many European countries. In a pooled analysis of 713 patients who participated in two randomized controlled trials (E1684 and E1690), HDI was associated with a significant reduction in relapse-free survival compared with observation (P <0.006).27 No benefit in overall survival was observed in the pooled analysis. The results of nine randomized clinical trials of adjuvant HDI or LDI versus observation in melanoma were included in a systematic review. The systematic review observed a trend toward reduced risk of recurrence of melanoma and of death among the IFN-treated patients in nearly all studies.30 Because of differences in dose, frequency, and duration of IFN-α treatment in the various trials, the review was not able to compare LDI versus HDI. Furthermore, the wide variability in number of patients enrolled, end points, patient selection, quality, type of therapy, duration of treatment, and follow-up precluded statistical analysis of the pooled results. Although the differences in overall survival were not always statistically significant, HDI remains the only adjuvant treatment shown to prolong survival in prospective randomized trials. IFN-α2b is approved by the United States FDA for treatment of patients with primary melanomas larger than 4 mm (stages IIB and IIC) and in patients with melanoma involving regional lymph nodes who are disease-free after lymph node dissection (stage III).
Although IFN is widely used in the adjuvant setting, there are concerns over the considerable treatment toxicities and the lack of consistent overall survival advantage of a toxic and expensive regimen. In addition, whether the results from the HDI trials should be extrapolated to patients with local recurrences, satellite lesions, or in-transit metastases is not clear. Remaining questions include the following: (a) Are the toxicities associated with HDI treatment worth the potential benefits for patients? (b) What are the mechanism(s) and best approaches to managing IFN toxicity? (c) Is the regimen or schedule of IFN used in the initial positive trial necessary to achieve the benefits seen in this study? Aggressive toxicity evaluation and individualized management are essential to help preserve quality of life in individuals receiving IFN therapy.
![]() A mechanism for optimizing the care of patients receiving IFN is to effectively prevent and manage treatment-related toxicities. A common syndrome seen with IFN-α therapy is a diverse group of side effects referred to as constitutional symptoms, which can include acute symptoms such as fever, chills, myalgia, and fatigue, and can encompass some of the more chronic toxicities such as fatigue, anorexia, and depression.31 Acetaminophen can be used to prevent or minimize acute dose-related symptoms such as fever, myalgia, and chills. Opiates, such as meperidine, are often required when patients experience severe chills or rigors, most commonly during the initial month of the HDI induction phase. Nonsteroidal antiinflammatory drugs (NSAIDs) have been used to manage IFN-related myalgia but may have overlapping side effects with IFN, such as a decrease in renal blood flow. Some NSAIDs, such as acetaminophen, may mask fevers that occur in patients who experience neutropenia while undergoing therapy. Additionally, NSAIDs may increase the risk of bleeding in the setting of thrombocytopenia caused by IFN. Fatigue is one of the most frequently observed dose-limiting toxicities seen with IFN therapy, occurring in 70% to 100% of patients.31 The mechanisms of IFN-induced fatigue are not fully understood and may be multifactorial in individual patients. IFN-induced fatigue appears to be dose related and may worsen with continued therapy. Pharmacologic (e.g., methylphenidate) and nonpharmacologic (e.g., exercise, psychosocial techniques, distraction, energy management, and dietary modifications) interventions for treatment of cancer-related fatigue and now IFN-related fatigue are being evaluated.31 Depression is common and should be fully evaluated. Contributing factors such as IFN-induced hypothyroidism or concomitant IFN symptoms (e.g., nausea and fatigue) should be evaluated concurrently with depression symptoms to optimize treatment decisions.32 Antidepressants, such as selective serotonin reuptake inhibitors, have been studied in IFN-induced depression with notable benefit.31 Anorexia was reported in about 70% of patients receiving adjuvant IFN therapy for melanoma and is thought to be mediated through direct effects on hypothalamic neurons, modification of normal hypothalamic neurotransmitters or neuropeptides, or effects from stimulation of other cytokines.31 Taste alterations may contribute to anorexia. Investigational strategies for ameliorating IFN-induced anorexia include nutritional intervention, use of appetite stimulants such as megestrol acetate, and patient education. Glucocorticoids should not be used for appetite stimulation or as part of an antiemetic therapy because they may adversely impact the immunomodulatory effects of IFN. Other toxicities such as hematologic or hepatic toxicities require monitoring and appropriate dose modification.
A mechanism for optimizing the care of patients receiving IFN is to effectively prevent and manage treatment-related toxicities. A common syndrome seen with IFN-α therapy is a diverse group of side effects referred to as constitutional symptoms, which can include acute symptoms such as fever, chills, myalgia, and fatigue, and can encompass some of the more chronic toxicities such as fatigue, anorexia, and depression.31 Acetaminophen can be used to prevent or minimize acute dose-related symptoms such as fever, myalgia, and chills. Opiates, such as meperidine, are often required when patients experience severe chills or rigors, most commonly during the initial month of the HDI induction phase. Nonsteroidal antiinflammatory drugs (NSAIDs) have been used to manage IFN-related myalgia but may have overlapping side effects with IFN, such as a decrease in renal blood flow. Some NSAIDs, such as acetaminophen, may mask fevers that occur in patients who experience neutropenia while undergoing therapy. Additionally, NSAIDs may increase the risk of bleeding in the setting of thrombocytopenia caused by IFN. Fatigue is one of the most frequently observed dose-limiting toxicities seen with IFN therapy, occurring in 70% to 100% of patients.31 The mechanisms of IFN-induced fatigue are not fully understood and may be multifactorial in individual patients. IFN-induced fatigue appears to be dose related and may worsen with continued therapy. Pharmacologic (e.g., methylphenidate) and nonpharmacologic (e.g., exercise, psychosocial techniques, distraction, energy management, and dietary modifications) interventions for treatment of cancer-related fatigue and now IFN-related fatigue are being evaluated.31 Depression is common and should be fully evaluated. Contributing factors such as IFN-induced hypothyroidism or concomitant IFN symptoms (e.g., nausea and fatigue) should be evaluated concurrently with depression symptoms to optimize treatment decisions.32 Antidepressants, such as selective serotonin reuptake inhibitors, have been studied in IFN-induced depression with notable benefit.31 Anorexia was reported in about 70% of patients receiving adjuvant IFN therapy for melanoma and is thought to be mediated through direct effects on hypothalamic neurons, modification of normal hypothalamic neurotransmitters or neuropeptides, or effects from stimulation of other cytokines.31 Taste alterations may contribute to anorexia. Investigational strategies for ameliorating IFN-induced anorexia include nutritional intervention, use of appetite stimulants such as megestrol acetate, and patient education. Glucocorticoids should not be used for appetite stimulation or as part of an antiemetic therapy because they may adversely impact the immunomodulatory effects of IFN. Other toxicities such as hematologic or hepatic toxicities require monitoring and appropriate dose modification.
Because of the associated toxicity and adverse effects seen with IFN-α therapy, many experts have questioned the usefulness of intensive adjuvant therapy for melanoma despite the possible benefits in relapse-free and overall survival. A subsequent report from the cooperative group study demonstrated a quality-of-life benefit with IFN therapy based on the quality-of-life–adjusted survival analysis.33 This analysis calculates the quality-of-life–adjusted years gained as a result of IFN-α treatment or the clinical benefit of time without toxicities and without disease. Another approach that has been investigated is the use of a pegylated product. Unfortunately, pegylated IFN has been evaluated in an attempt to improve the benefit-toxicity ratio without much success.34
![]() The role of IFN as adjuvant therapy is not clear at this time. If adjuvant IFN is given, it is not clear what product (e.g., pegylated IFN), dose, and duration of therapy should be used. The issues of patient side effects and cost must be carefully weighed against the potential disease-free survival benefit. Because HDI is the only therapy to demonstrate benefit in large comparative trials, it should be considered for patients with high-risk disease. The 2013 NCCN guidelines for melanoma list IFN-α as one of several options for select patients with high-risk disease.20 Other options include observation and probably, most importantly, clinical trials. Individuals should be prescreened for potential problems associated with therapy; relative contraindications to HDI therapy include autoimmune diseases, immunosuppression, decompensated liver disease, severe neuropsychiatric diseases, and life-threatening infection.31 Efforts continue to better define the optimal treatment regimen for HDI versus other strategies in well-designed clinical trials.
The role of IFN as adjuvant therapy is not clear at this time. If adjuvant IFN is given, it is not clear what product (e.g., pegylated IFN), dose, and duration of therapy should be used. The issues of patient side effects and cost must be carefully weighed against the potential disease-free survival benefit. Because HDI is the only therapy to demonstrate benefit in large comparative trials, it should be considered for patients with high-risk disease. The 2013 NCCN guidelines for melanoma list IFN-α as one of several options for select patients with high-risk disease.20 Other options include observation and probably, most importantly, clinical trials. Individuals should be prescreened for potential problems associated with therapy; relative contraindications to HDI therapy include autoimmune diseases, immunosuppression, decompensated liver disease, severe neuropsychiatric diseases, and life-threatening infection.31 Efforts continue to better define the optimal treatment regimen for HDI versus other strategies in well-designed clinical trials.



