Medication Absorption in Bariatric Surgery
Branden D. Nemecek
Outline
•Introduction
•Roux-en-Y Gastric Bypass
•Effect of Additional Bariatric Surgeries on Medications
•Summary Table: Pharmacokinetic Changes Post-RYGB
•Summary Table: Medication Levels Post-RYGB
Bariatric surgeries have become common due to the high rates of obesity worldwide and successful weight loss demonstrated from the procedures. These surgeries are categorized as either restrictive, malabsorptive, or a combination of the two. Restrictive surgeries involve the creation of a small gastric pouch with a goal of increasing early satiety and a reduction of nutritional intake.1 These procedures delay gastric emptying but have no effect on the size of the absorptive area of the intestines. Weight loss is due to increased early satiety and decreased nutritional intake. Examples of restrictive surgeries include laparoscopic gastric banding or a gastric sleeve.1 Malabsorptive surgeries involve the reduction of the size of the absorptive area of the intestines, leading to less time and area for nutrient absorption to occur.1 The weight loss in these procedures is believed to be due to decreased nutrient absorption rather than decreased nutritional intake. Procedures such as Roux-en-Y gastric bypass (RYGB) were originally classified as malabsorptive surgeries but are now believed to be a combination of restrictive and malabsorptive in nature.1
Laparoscopic RYGB is considered one of the more successful weight-loss surgeries and is the most frequently performed bariatric surgery, accounting for about 80% to 90% of all weight-loss procedures performed in the United States.2,3 This type of bariatric procedure is categorized as both restrictive and malabsorptive.4 The RYGB procedure involves the creation of a small pouch (≤50 mL) at the stomach along with the creation of the Roux limb attaching the proximal portion of the stomach to the central portion of the small intestine, delaying the exposure of food to bile and pancreatic juices.2,3 This procedure completely bypasses the duodenum and reduces the stomach capacity by 95%, leaving the majority of the jejunum intact.2,3,5 Unlike biliopancreatic diversion and jejunoileal bypass, RYGB leaves approximately 4 meters of the small intestine still intact.5
Jejunoileal bypass is a procedure similar to that of RYGB in that it is a restrictive and malabsorptive in nature, although this procedure is no longer recommended due to significant adverse events and mortality. In this procedure, approximately 90% to 95% of the small intestine is bypassed with no reduction in stomach size.1 Very few patients retain this surgery due to the mortality rates or because they have undergone conversion via a different procedure.1
Laparoscopic adjustable gastric banding is a procedure in which a band is placed near the gastroesophageal junction restricting oral intake by limiting the size of the stomach.1 This creates a pouch of approximately 30 mL, although the size of this pouch can be modified through injection of saline into the balloon that surrounds the band.1 The procedure for a sleeve gastrectomy involves stapling of the stomach, resecting a significant portion of the stomach.1 Laparoscopic banding and sleeve gastrectomy are considered to be restrictive surgeries, although sleeve gastrectomy may have some malabsorptive properties. Restrictive procedures are hypothesized to have minimal effect on overall drug absorption; however, changes in gastric mixing and possibly pH have been noted.6
Roux-en-Y Gastric Bypass
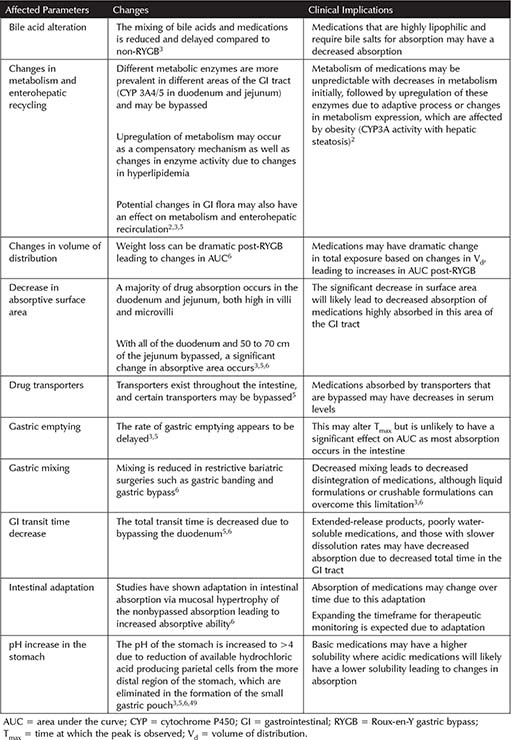
The overall effect of the RYGB procedure on pharmacokinetics (PK) is unknown. Multiple implications on drug absorption have been hypothesized, including the effect on surface area for absorption, gastrointestinal (GI) transit time, pH of the stomach, rate of gastric emptying, gastric mixing, changes in metabolism, and alteration of drug transporters. These factors are discussed in Table 10-1.
Recommendations on medication therapy in patients post-RYGB are limited. In general, due to the changes discussed in Table 10-1, liquid formulations or those that can be crushed or chewed are recommended when available.6 Due to changes in pH and GI transit time, extended- or controlled-release products are not recommended after RYGB.5 Studies on RYGB are relatively small and often do not include clinical outcomes or clinical correlation. Therapeutic recommendations below are therefore limited based on minimal published evidence.
Antibiotics
Amoxicillin
A case report of a pregnant female, who had previously undergone RYGB, failed amoxicillin therapy for treatment of a urinary tract infection that was susceptible to amoxicillin.7 The authors questioned adequate absorption of the medication. This report states that the patient was compliant with therapy when treatment failure occurred. No PK parameters were provided in this report.
Table 10-1. Potential Effects of RYGB on Medication Pharmacokinetics
The PK of a single dose of oral azithromycin appears to be altered by RYGB through a statistically significant reduction in area under the curve (AUC) of 32% when compared to a single dose administered to a BMI-matched control group (p = 0.008).8 The dose normalized to AUC also showed a significant decrease of 33% (p = 0.009). Decreases in Cmax (peak serum concentration) of 28% and Tmax (time at which the peak is observed) of 9% were noted after surgery, but these changes were not statistically significant. The decrease in AUC and Cmax are hypothesized to be due to the decreased absorptive area post-RYGB as azithromycin has been previously shown to be primarily absorbed in the upper proximal gut. There is a potential for treatment failures with azithromycin based on the decreased AUC and Cmax post-RYGB. The levels achieved in this study are believed to be adequate for treatment of most organisms, but those organisms with increased minimum inhibitory concentration (MIC) may experience treatment failure. Clinical evidence is lacking to support utilizing an increased dose, so patients receiving azithromycin post-RYGB should have close monitoring for treatment failure and alternative therapy may be considered.
Erythromycin
Erythromycin PK parameters postbariatric surgery have been reviewed through a case report.9 In this case, there was a 100% increase in Tmax, a 22% decrease in Cmax, and a 57% increase in AUC. No clinical correlation was noted in this study. It is hypothesized that delayed gastric emptying led to slower but increased absorption. This increase in AUC may lead to increase adverse reactions such as QTc prolongation, thus monitoring for adverse effects is warranted.
Linezolid
Oral linezolid was found to have a significantly increased AUC, 3 months post-RYGB with an increase of 138% (p <0.001).10 The remaining PK parameters studied were not significantly different, although the Cmax did increase 29%. Intravenous (IV) linezolid was also reviewed and showed similar changes in PK parameters as oral therapy. With similar PK parameters between oral and IV therapy, it was hypothesized that linezolid absorption does not appear to be altered by RYGB surgery, but rather the PK changes are due to the median weight loss of 30.9 kg potentially affecting Vd. Due to no significant change in PK parameters of linezolid post-RYGB, no dose adjustments are recommended at this time.
Moxifloxacin
Oral moxifloxacin has been shown to maintain a 1:1 bioequivalence ratio compared to IV moxifloxacin post-RYGB as there was not a statistically significant difference between the oral and IV formulation’s PK profiles.11 Interestingly, the overall AUC was found to be approximately 51% higher for the IV and 54% higher for the oral formulation after RYGB compared to studies in healthy volunteers. In addition, Cmax increased by 25% with IV therapy and 35% with oral therapy. Previous PK studies with moxifloxacin have shown different AUC depending on if they are studied in men or women. The RYGB study was predominately females, making application of this information to men difficult. It is hypothesized that changes in enterohepatic recirculation due to a more distal secretion of bile and pancreatic fluids after surgery may result in an increased AUC for moxifloxacin. With the evidence provided, moxifloxacin appears to have good bioavailability after RYGB, and no dose adjustments are necessary.
A pregnant female, who had previously undergone RYGB, failed amoxicillin therapy for treatment of a urinary tract infection that was susceptible to amoxicillin.7 Adherence to the therapy was reported, but no levels or PK data were acquired. The use of nitrofurantoin post-RYGB needs to be further evaluated, as there is only a case report of treatment failure.
Summary 
•Additional studies are warranted on antibiotics due to the clinical implications of changes in PK in agents where exceeding the MIC is imperative.
•Dose adjustments do not appear to be necessary for moxifloxacin and linezolid.
•Azithromycin utilization at institutions with microbes having higher MIC may experience treatment failure. Alternative agents may be considered, and close monitoring of efficacy is necessary.
•Nitrofurantoin and amoxicillin have documentation of treatment failure, although strong evidence is lacking.
•Erythromycin demonstrates an increase in exposure leading to increased monitoring for adverse events such as QTc prolongation or hepatotoxicity.
Antivirals
Darunavir
In a case report evaluating the PK parameters of darunavir 300 mg orally twice daily, an observed trough of 2,602 ng/mL post-RYGB was comparable to published troughs in the non-RYGB population.12 Previously reported troughs had a range of 1,517 to 13,198 ng/mL with a median of 3,307 ng/mL. Based on this single case report, it does not appear as if the dose of darunavir needs to be adjusted post-RYGB.
Emtricitabine
In a case report of emtricitabine 200 mg daily, an observed trough of 57 ng/mL post-RYGB was comparable to previously published trough levels in non-RYGB patients between 47 and 112 ng/mL.12 Based on this single report, emtricitabine dosing does not need to be adjusted post-RYGB.
Lamivudine
A case report demonstrated no significant changes in the peak and trough concentration from pre- to post-RYGB surgery, although both values were stated as below the reference range.13 Based on these peaks and troughs, PK data post-RYGB do not appear to be affected, and standard dosing can be utilized.
Nelfinavir
In the aforementioned case report, a decrease in both peak and trough concentrations 180 days after RYGB surgery was found for nelfinavir.13 The postsurgery levels at 14 days were a peak of 1 mcg/mL and trough of 0.4 mcg/mL. M8, a metabolite of nelfinavir, was also shown to be decreased post-RYGB surgery from a presurgical peak of 0.31 mcg/mL to undetectable levels. Due to this dramatic change, use of nelfinavir after RYGB is not recommended until further studies are performed.
A case report of ritonavir 100 mg orally twice daily observed a trough of 54 ng/mL post-RYGB, which was comparable to published data in non-RYGB patients with a mean trough of 308 ± 242 ng/mL.12 Based on this report, it does not appear the dose of ritonavir as a boosting agent needs to be adjusted post-RYGB as the boosted darunavir levels were still well within therapeutic range.
Tenofovir
A case report of tenofovir 300 mg orally daily led to an observed trough of 40 ng/mL post-RYGB. This value was comparable to previously published troughs in non-RYGB patients ranging from 30.6 to 140 ng/mL.12 Tenofovir dosing does not need to be adjusted post-RYGB.
Zidovudine
A case report showed no significant changes in the peak and trough concentration pre- and post-RYGB surgery, although both values were below reference ranges.13 Only the pre-RYGB levels were provided with a peak of 0.4 mcg/mL and trough of 0.02 mcg/mL. The Tmax was reduced in this case by 28%. Based on the lack of change in PK levels post-RYGB, dose adjustments are not recommended.
Additional Antivirals
Additional antiretroviral therapies have been reported as being effective in patients after RYGB.12 These agents did not have PK parameters documented but maintained clinical efficacy after surgery. The agents included in successful combinations include stavudine, didanosine, efavirenz, raltegravir, tenofovir, and emtricitabine.
Summary 
•Nelfinavir displays subtherapeutic levels after RYGB, and alternative agents should be strongly considered.
•No dose adjustments appear necessary for emtricitabine, darunavir, ritonavir, and tenofovir based on post-RYGB levels within therapeutic ranges.
•Lamivudine and zidovudine do not appear to be affected by RYGB, although levels before and after RYGB were below reference ranges making application of data difficult.
•Stavudine, didanosine, efavirenz, raltegravir, and tenofovir have been successfully utilized after RYGB surgery, although no PK data are available for these agents.
Immunosuppressants and Immunomodulators
Hydroxychloroquine
Hydroxychloroquine showed a dramatic increase in Cmax and AUC at 1 month post-RYGB, and these parameters remained elevated at 12 months, although they had decreased closer to presurgical levels.14 The Cmax increased by 258% at 1 month and by 108% at 12 months compared to presurgical levels, with the AUC increasing by 92% and 12%, respectfully. The increased Cmax was above the published reference ranges, while AUC increased but was within ranges previously published. Due to an elevated Cmax and AUC, toxicity such as optic neuritis should be monitored closely, and dose reductions may be necessary.
Methotrexate initially showed similar PK parameters compared to presurgical patients at 1 month, but at 12 months the Cmax had decreased by 86% and the AUC by 66%.14 The levels at 12 months were dramatically lower than therapeutic values previously reported in the literature. In this case report, the patient experienced clinical deterioration of the disease state in correspondence with the decrease in methotrexate levels, which resolved with the conversion from oral to subcutaneous methotrexate therapy. It is hypothesized that changes in efflux transporters within the GI tract may contribute to this clinically significant change.
Methotrexate levels should be monitored at normal intervals for those using methotrexate for indications with a known therapeutic window as this report showed time-
dependent changes in absorption after surgery. It is unknown if the data from this single case report are applicable to the general population.
Mycophenolic Acid
Mycophenolic acid PK parameters showed dramatic inter- and intrapatient variability in six patients who underwent RYGB.15 Clinically significant differences such as a 57% lower AUC were noted in the four end-stage renal disease (ESRD) patients (24-hour dosing schema, not at steady state) who underwent RYGB surgery and were about to undergo kidney transplant (continuous, at steady state) compared to previously published data on nonbariatric surgery patients. The two kidney recipients had a 35% lower AUC than the general population in previously published data. The lower AUC noted in the RYGB population is hypothesized to be due to the alteration in enterohepatic recirculation, reducing the second mycophenolic acid peak. In the kidney transplant recipients who underwent RYGB, a higher AUC was noted compared to ESRD patients who underwent RYGB surgery. A lower Cmax was seen in the ESRD arm than kidney transplant, but these results were similar to previous studies in ESRD, so these PK changes are more likely due to ESRD than RYGB.
Based on the evidence in this study, patients likely will need increased monitoring due to variability in absorption and the noted decreased AUC. Increased doses may be necessary in some, but not all patients, due to interpatient variability.
Prednisone
The Cmax and AUC of prednisone and its active metabolite, prednisolone, were found to be decreased at 1-month post-RYGB surgery.14 At 12 months, these levels remained lower but had begun to trend back closer to RYGB preoperative levels. The Cmax decreased by 42% at 1-month post-RYGB and 24% at 12-months postoperative. The AUC values decreased by 43% at 1-month postsurgery and by 38% at 12 months post-RYGB, which shows that at 1-month post-RYGB, the PK parameters are below reference ranges, while at 12 months, the Cmax was within range. The AUC was still below the reference range at both 1-month and 12-month time points. It is hypothesized that the changes over time after RYGB are due to changes in efflux transporters in the distal intestine, such as p-glycoprotein. Prednisone absorption and exposure is decreased post-RYGB surgery but over time may improve to near baseline. With this change in absorption initially, there is a potential need for increased dosing.
Sirolimus
In the aforementioned study of six patients, including four ESRD patients who were about to undergo kidney transplant and two patients who had already underwent kidney transplant, significant interpatient variability was observed in all PK parameters (Cmax and AUC) except for Tmax.15 Compared to evidence in healthy volunteers using liquid sirolimus, the Tmax increased by 115% to 186%, but there was a decrease in Cmax ranging from 43% to 60%. The reduction in AUC was estimated between 46% to 60% and the AUC/dose ratio (normalization of AUC to the test dose) decreased by 37% to 47%.
Sirolimus absorption occurs primarily in the proximal duodenum, similarly to tacrolimus, explaining why levels were significantly decreased. 15 The bypassed duodenum is also the primary site of metabolism (CYP3A4/5). Based on this, patients may need increased doses to achieve therapeutic levels after RYGB. Due to the limited evidence, no specific recommendations can be made, but increased monitoring for efficacy is indicated in patients on sirolimus post-RYGB.
Tacrolimus
In the previously mentioned study of four ESRD patients who were about to undergo kidney transplant and two patients who had already undergone kidney transplant, there was large interpatient variability in PK parameters.15 In comparison to previous studies in healthy volunteers, a lower dose/kg was analyzed in the post-RYGB population. There were slight variations in Tmax and Cmax
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


