Figure 6.1
DeBakey and Stanford classification of aortic dissection. The DeBakey classification system categorizes dissections based on the origin of the intimal tear and the extent of the dissection: Type I: Dissection originates in the ascending aorta and propagates distally to include at least the aortic arch and typically the descending aorta. Type II: Dissection originates in and is confined to the ascending aorta. Type III: Dissection originates in the descending aorta and propagates most often distally. Type IIIa: Limited to the descending thoracic aorta. Type IIIb: Extending below the diaphragm. The Stanford classification system divides dissections into two categories, those that involve the ascending aorta and those that do not. Type A: All dissections involving the ascending aorta regardless of the site of origin. Type B: All dissections that do not involve the ascending aorta. Note involvement of the aortic arch without involvement of the ascending aorta in the Stanford classification is labeled as Type B (Reproduced with permission from Nienaber et al. [1])
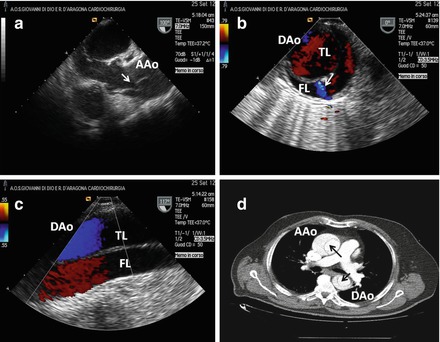
Figure 6.2
Chronic Stanford A aortic dissection in 68 years old man with history of hypertension and chest pain occurred 2 months before hospital admission. (a) Transesophageal echocardiography (TEE) in long axis view demonstrating intimal flap (see arrow) in ascending aorta (AAo). (b) and (c) TEE in short and long axis view respectively of the descending aorta (DAo) showing anterior true lumen (TL) and posterior false lumen (FL); note a distal small intimal tear (b, see arrow). (d) Computed tomography of the aorta: intimal flap (see black arrows) in both ascending and descending tract can be appreciated
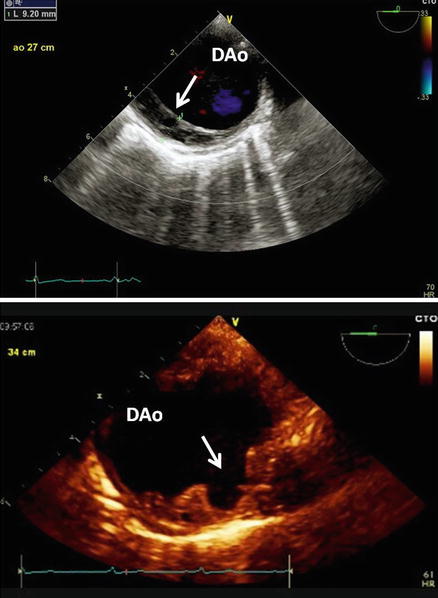
Figure 6.3
Top: TEE short axis view of the DAo. Note the abnormal thickness of posterior wall do to chronic intramural haematoma. Bottom: Penetrating ulcer of atherosclerotic plaque can be clearly appreciated as an incidental finding in a patient with history of hypertension and diabetes mellitus
Given the high risk of complications and non-specific symptoms and signs AAS requires a high clinical index of suspicion. Prompt diagnosis and appropriate therapeutic interventions are paramount to enhance survival [1, 3]. However, after the acute phase, AAS persist with a high risk of re-dissection, aneurysm formation, and/or rupture.
Long-Term Outcomes
The 10-year survival rate of patients with an aortic dissection may range from 30 to 60 % [1, 4–14]. Among 303 consecutive cases with TA-AAD enrolled in the International Registry of Acute Aortic Dissection (IRAD) (90.1 % managed surgically vs 9.9 % medically), survival for patients treated with surgery was 96.1 % ± 2.4 % and 90.5 % ± 3.9 % at 1 and 3 years versus 88.6 % ± 2.2 % and 68.7 % ± 19.8 % without surgery (mean follow-up overall, 2.8 years) [12] (Fig. 6.4). History of atherosclerosis and previous cardiac surgery were identified as independent predictors of follow-up mortality [12]. On the other hand, 3-year survival for type B aortic dissection patients (n = 242) treated medically, surgically, or with endovascular therapy was 77.6 ± 6.6 %, 82.8 ± 18.9 %, and 76.2 ± 25.2 %, respectively (median follow-up 2.3 years) [13] (Fig. 6.5). In that series, independent predictors of follow-up mortality included female sex, history of aortic aneurysm, history of atherosclerosis, in-hospital renal failure, pleural effusion on chest radiograph, and in-hospital hypotension/shock [13]. Some clinical predictors of complications, such as Marfan syndrome [15–17], age [17, 18], chronic obstructive pulmonary disease [17] or atherosclerotic disease have been reported [19]. In addition, maximum descending aorta diameter [12, 17, 20–22], true lumen compression or large false lumen diameter, partial false lumen thrombosis and the presence of a large proximal entry tear [23] are predictors of mortality and the need for surgical/endovascular treatment. Patent false lumen in descending aorta segments after surgical treatment of type A dissection is frequent (64–90 %) [18, 24, 25]. Suboptimal connection of the distal part of the graft implanted in ascending aorta to the true lumen or presence of secondary tears may account for the persistence of flow into the distal residual false lumen after complete surgical resection of the primary entry tear. Long-term outcome of aortic dissection with patent false lumen in descending aorta presents a higher risk of complications in type B than in type A dissections, particularly after 3 years of evolution. The expansion rate of the chronic dissected aorta is not particularly well characterised, but ranges between 0.1 and 0.7 cm per year.
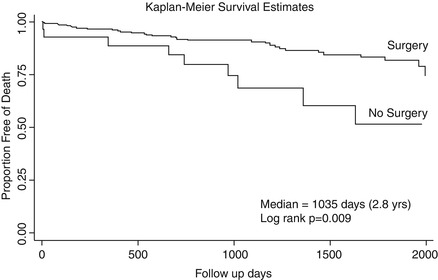
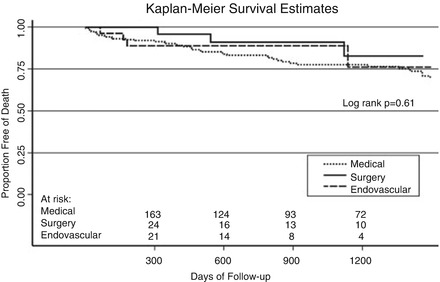

Figure 6.4
Unadjusted Kaplan-Meier survival curve stratified by in-hospital management from date of hospital discharge. This figure shows the survival curves estimated by the Kaplan-Meier method stratified by in-hospital management. The unadjusted survival rate at 1 year was 96.1 % ± 2.4 % and 88.6 % ± 12.2 % for surgery versus medical treatment, respectively, with further separation of the curves at 3 years with survival rates of 90.5 % ± 3.9 and 68.7 % ± 19.8 (median 2.8 years, log rank P = 0.009) (Reproduced with permission from Tsai et al. [12])

Figure 6.5
Unadjusted Kaplan-Meier survival curve stratified by in-hospital management. This figure shows the survival curves estimated by the Kaplan-Meier method stratified by in-hospital management. The unadjusted survival rate at 1 and 3 years for patients discharged from the hospital alive was 90.3 ± 4.3 % and 77.6 ± 6.6 % for medical therapy alone, 95.8 ± 8.0 % and 82.8 ± 18.9 % for surgery, and 88.9 ± 11.9 % and 76.2 ± 25.2 % for endovascular treatment (median 2.3 years, log-rank P = 0.63) (Reproduced with permission from Tsai et al. [13])
Evangelista et al. investigated the long-term clinical and morphological evolution of 50 IMH. In the first 6 months, total IMH regression was observed in 14 and progression to aortic dissection in 18 patients; in 14 of these, the dissection was localised, and 12 later developed pseudoaneurysm. At the end of follow-up (mean: 45 ± 31 months), the IMH had regressed completely without dilatation in 17 patients (34 %), progressed to classical dissection in 6 (12 %), evolved to fusiform aneurysm in 11 (22 %), evolved to saccular aneurysm in 4 (8 %), and evolved to pseudoaneurysm in 12 (24 %) [14] (Fig. 6.6). Multivariate analysis showed an independent association between regression and smaller maximum aortic diameter and between aneurysm formation and atherosclerotic ulcerated plaque and absence of echolucent areas in IMH [14].
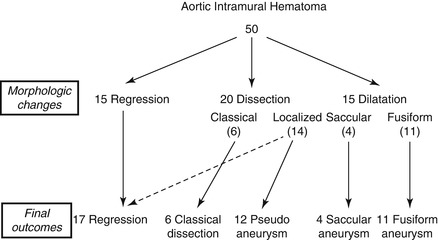

Figure 6.6
Different evolution patterns of IMH from morphological changes to final outcomes (Reproduced with permission from Evangelista et al. [14])
Imaging Surveillance
The patient with AAD demands careful clinical and imaging monitoring by a specialised aorta team in order to detect signs of aortic expansion/dissection, aneurysm formation, leakages at anastomosis/stent sites, and malperfusion [2, 28]. Computed tomography (CT) and magnetic resonance imaging (MRI) providing a comprehensive evaluation of the aorta represent ideal tools for serial imaging [2, 26] (Table 6.1).
Table 6.1
Relative strengths of imaging modalities for acute aortic syndromes
TTE | TEE | MRI | CT | |
|---|---|---|---|---|
Imaging factors | ||||
Comprehensive aortic assessment | + | ++ | +++ | +++ |
Tomographic (3D reconstruction) | – | – | +++ | +++ |
Functional | +++ | +++ | ++ | + |
Tissue characterization | – | – | +++ | +++ |
Clinical factors | ||||
Portability | +++ | ++ | – | – |
Patient access/monitoring | +++ | +++ | + | ++ |
Rapidity | ++ | ++ | ++ | +++ |
Non-contrast | +++ | +++ | + | + |
Radiation exposure | +++ | +++ | +++ | + |
MRI, although not widely available, should be considered the technique of choice. In fact, it provides tomographic-3D reconstruction, tissue characterisation and functional assessment. It entails no radiation exposure and minimises the risk associated with the use of gadolinium – based contrast agents (excellent safety profiles). However, gadolinium-based contrast agents are contraindicated in patients with advanced renal impairment (glomerular filtration rate <30 mL/min) owing to risks of nephrogenic systemic fibrosis. MRI is prohibited in patients with ferromagnetic and/or magnetically-activated implants (including most cardiac pacemakers, defibrillators) and image artifact can interfere with the assessment of vascular stents [26–29].
Current guidelines recommend: (a) regular outpatient visits and imaging at 1, 3, 6, 9 and 12 months post-dissection and annually thereafter, depending on aortic size and the patient’s clinical condition (hypertension and aortic expansion/dissection are common early after discharge), and (b) to utilise for each patient the same modality at the same institution so that similar images can be compared side by side [2, 27] (Table 6.2).
Table 6.2
Imaging follow-up of aortic pathologies after repair or treatment
Pathology | Interval | Study |
|---|---|---|
Acute dissection | Before discharge, 1 month, 6 months, yearly | CT or MR, chest plus abdomen TTE |
Chronic dissection | Before discharge, 1 year, 2 to 3 years | CT or MR, chest plus abdomen TTE |
Aorticroot repair | Before discharge, yearly | TTE |
AVR plus ascending | Before discharge, yearly | TTE |
Aorticarch | Before discharge, 1 year, 2 to 3 years | CT or MR, chest plus abdomen |
Thoracicaortic stent | Before discharge, 1 month, 2 months, 6 months, yearly or 30 daysa | CXR, CT, chest plus abdomen |
Acute IMH/PAU | Before discharge, 1 month, 3 months, 6 months, yearly | CT or MR, chest plus abdomen |
Studies have suggested detection of increased FDG uptake (marker of active inflammation) by positron emission tomography (PET)/CT may help to differentiate acute from chronic AAD. The combination of PET/CT and vascular/aortic biomarkers that reflect remodelling (e.g. transforming growth factor α [TGF-α]) may have potential risk prediction value during the following of AAS patients [30–32]. In addition, plasma MMP levels might also be used in long-term follow-up to monitor aortic remodelling [30, 33]. However, further studies are needed to explore potential clinical applications of biomarkers in chronic aortic dissection [30].
Medical Treatment (Table 6.3)
Table 6.3
Medical treatment and lifestyle goals in the follow-up of patients with chronic aortic syndrome [2]
Medical treatment |
1. Optimal blood pressure < 120/80 mmHg and heart rate < 60 bpm control |
First line: beta-blockers |
Second line: ACE inhibitors or ARB |
Third line: calcium channel blockers (long-acting dihydropyridine) |
2. Lipid lowering therapy: target of LDL cholesterol less than 70 mg/dL |
Lifestyle goals |
Low-fat and low-salt diet |
Achieve an ideal body weight |
Smoking cessation (special programs, and/or pharmacotherapy, including nicotine, replacement, buproprion, or varenicline may be useful) |
Avoid cocaine or other stimulating drugs such as methamphetamine |
Avoid strenuous physical activities, isometric exercise, pushing, or straining that would require a Valsalva maneuver |
Avoid contact sports that can cause sudden stress or trauma to the thorax, (e.g. competitive football, ice hockey, or soccer etc.) |
Mild aerobic exercise and daily activities are not restricted |
Adherence to medical treatment |
Optimal Blood Pressure Control
Hypertension represents one of the key causative factors of AAS [2, 34]. Patients with AAS often require the combination of at least two drugs to achieve blood pressure and heart rate control [35]. On the basis of the data from patients with Marfan’s syndrome, long-term beta blockade (negative inotropic and chronotropic effects, lower blood pressure and decreased dp/dt) is usually recommended in patients with aortic dissection to maintain blood < 120/80 mmHg and heart rate < 60 bpm (first line) [2, 36–39]. Genoni et al. reported improved survival in patients treated with beta-blockers 1685 in the chronic phase of aortic dissection [40]. That study observed an 80 % freedom from aortic events at a mean of 4.2 years in patients on beta-blockers, in comparison with 47 % freedom from aortic events in patients treated with other anti-hypertensive agents. The efficacy of other antihypertensive drugs has not been demonstrated in patients with chronic type B aortic dissection although they have a role in maintaining the patient’s blood pressure at the appropriate level. Long-acting rather than short acting beta-blockers should be preferred to reduce side effects and increase compliance. Observational studies suggest similar or better benefits in aortic dissection when compared with other antihypertensive agents [11, 41]. Guidelines recommend progressive uptitration of dosage to achieve a blood pressure 135/80 mmHg in usual patients and 130/80 mmHg in those with Marfan syndrome [42, 43], Several studies have suggested that between 40 and 70 % of late deaths in patients with chronic aortic dissection are non-aorta related and due to comorbid diseases, mainly heart disease and stroke [15, 44], thus implying that cardiovascular risk factors should be thoroughly assessed in this group. Interestingly, cigarette smoking seems not to affect the expansion and rupture rate of chronic type B aortic dissection, although its detrimental role in cardiovascular risk is well established. Although their role in the incidence of late aortic complications has not been demonstrated, cardiovascular risk-reduction measures (such as cholesterol treatment, anti-platelet therapy, management of hypertension and smoking cessation) is advisable for patients with chronic dissections to reduce the incidence of late cardiovascular death.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


