Figure 11.1
Pooled risk ratios for various end points from six randomised controlled trials comparing percutaneous transluminal coronary angioplasty (PCTA) with medical treatment in patients with non-acute coronary heart disease; (CABG: coronary artery bypass grafting; n = 953 for PTCA and 951 for medical treatment) (*Reproduced with permission from Bucher et al. [16])
The post–stent period led to a dramatic decrease in restenosis rates and the need for repeat PCI [20–22]. This is one of the key reasons that explain the exponential growth and use of interventional techniques in recent years. Importantly though, there have also been advances during this period, in the pharmacological treatment of patients with chronic ischemic heart disease [16, 21]. Two studies are ground breaking in this regard: the BARI 2D [23] and COURAGE [24] trials (Table 11.1). BARI 2D was designed to compare, in patients with type 2 diabetes mellitus, the effects of PCI versus coronary-artery bypass grafting (CABG), both on top of optimal medical therapy (OMT), versus OMT alone. 2,368 patients with documented ischemia who were either asymptomatic or who had mild to moderate symptoms were included in the study. No difference was seen regarding 5-year mortality between revascularization and OMT alone (11.7 % versus 12.2 %; P = 0.97) (Fig. 11.2). Similarly, no difference was observed in 5-year rates of the combined end point of death, myocardial infarction, and stroke (22.8 % versus 24.1 %; P = 0.70) [23]. The COURAGE trial [24] is the largest study so far comparing the results of interventional therapy combined with optimal medical therapy (intensive pharmacological treatment and lifestyle change) with optimal medical therapy alone in patients with chronic ischemic heart disease. The trial involved 2,287 patients, who were randomized to PCI combined with medical treatment (1,149) and to medical treatment alone (1,138). The primary outcome was death from any cause or non-fatal infarction during the follow-up period (Fig. 11.3). The 4.6-year cumulative rates showed no significant differences in the primary outcome between the PCI group and the medical-therapy-alone group (19–18.5 %; P = 0.62). This similarity remained when peri-procedural myocardial infarction was excluded (16.2–17.9 %; P = 0.29). No differences were observed either in the incidence of hospitalization for acute coronary syndrome or in the mortality rates of both groups. Between the first and third year of follow-up, there was a substantial reduction of the prevalence of angina in both groups, but there was a significant difference in favour of the PCI group (55–66 % after 1 year, and 67–72 % after 3 years). These differences disappeared during the fifth year of follow-up (approximately 73 % of patients in both groups were free from angina). Patients who underwent PCI had less need for additional revascularization (21.1 % of patients in the PCI group had to undergo bypass surgery, as compared with 32.6 % of the medical-therapy group; P < 0.001). In summary, the COURAGE trial suggested that, in comparison with medical therapy alone, optimal medical therapy combined with PCI (mainly with conventional stents) showed similar death and infarction rates, but with less need for revascularization and a better initial control of angina [24, 25]. Thus the BARI-2D and COURAGE trials addressed a crucial question, with both trials demonstrating that revascularization is not superior to OMT regarding reductions in mortality or major cardiovascular events [26].
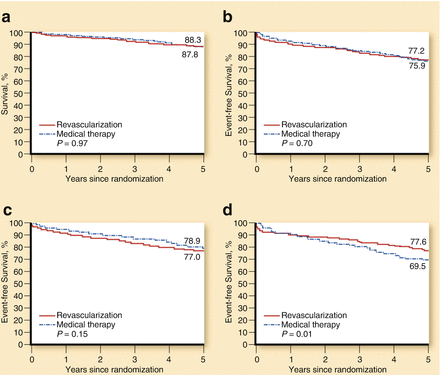
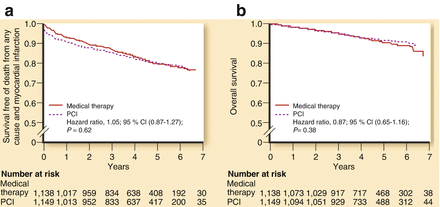
Table 11.1
Baseline clinical characteristics in COURAGE and BARI-2D trials
Courage | BARI-2D | |
|---|---|---|
Year of initial publication | 2008 | 2009 |
Period of recruitment | 1999–2004 | 2001–2005 |
Entry criteria | CAD by catheter plus positive stress or angina | CAD by catheter plus T2DM plus positive stress or angina |
Type of revascularization and randomization | PCI + OMT vs OMT | PCI + OMT vs OMT CABG + OMT vs OMT |
Primary end point | Death/nonfatal MI | Death |
Secondary end point | Death/MI/stroke/ hospitalization for unstable angina | Death/MI/stroke |
Follow-up, y | 4.6 | 5 |
Patients, n | 2,287 | 2,368 |
OMT patients, n | 1,149 | 1,192 |
Revascularization patients, n | 1,138 | 1,176 (798 for PCI, 378 for CABG) |
Mean age, y | 61 | 62 |
Previous MI | 38 % | 32 % |
T2DM | 34 % | 100 % |
Angina CCS classification | 78 % with 0–II | 60 % with 0–II |
21 % with III | 9 % with III–IV | |
Mean LVEF | 61 % | 57 % (82 % were normal) |
Crossover (medically treated and revascularized during follow-up) | 33 % | 42 % |

Figure 11.2
Major clinical endpoints in the BARI-2D trial. (a) Survival: revascularization vs medical therapy. (b) Freedom from major cardiovascular events: revascularization vs medical therapy. (c) Freedom from cardiovascular events in the PCI stratum. (d) Freedom form cardiovascular events in the CABG stratum (Reproduced with permission from Fernandez and Boden [26])

Figure 11.3
Major clinical end points in the COURAGE trial. (a) Survival free of death from any cause and myocardial infarction. (b) Overall survival (Reproduced with permission from Fernandez and Boden [26])
Limitations of These Studies
These two trials have limitations that should be considered. One of these is the percentage of crossover towards revascularization in the medical treatment group. In COURAGE, crossover over 5 years of follow up occurred in 33 % of cases while it was expected to occur in only 5 %. In the BARI 2D trial, rate of crossover to revascularization was 42 %. Another important point is the lack of quantification of the ischemic area. Moreover, drug-eluting stents were not widely available when these studies started, so bare metal stents mainly were used. We can only speculate that this could have had an impact on clinical outcomes. Finally, many high- risk patients were directly sent to revascularization after coronary angiography without randomization.
Revascularization Options: PCI Versus CABG
Before the more recent evolution of stents, several randomized studies (SoS, MASS-II, BARI, AWSOME, ARTS) compared the efficacy of PCI and CABG in patients with multivessel SCAD [27–31]. Similar rates of mortality were observed with both strategies, except in diabetic patients, who had better results with surgery in the BARI trial. According to these studies, stroke and non-fatal myocardial infarction rates were also similar in both treatment arms, with patients undergoing PCI needing more repeat revascularization procedures. In the drug eluting stent (DES) era, the ARTS I study was designed to compare CABG with stenting in patients with multivessel disease. At 5 years of follow up there were no differences in mortality between stenting and CABG. The ARTS II study studied the 5-year safety and effectiveness of the sirolimus eluting stent in patients with multivessel disease and compared the outcomes of ARTS II with the outcomes of the 2 historical arms of ARTS I. At 5 years, sirolimus eluting stent had a safety record comparable to CABG and superior to bare metal stents, and a MACE rate that was higher than that in patients treated with CABG, but lower than that in patients treated with bare metal stents. There were no differences in mortality between stenting and CABG [32, 33].
The SYNTAX trial randomized 1,800 patients with three-vessel disease or left main coronary artery disease to CABG or paclitaxel DES. One novelty introduced in this study was the use of an angiography score to grade the complexity of coronary artery disease; the “SYNTAX score” (Fig. 11.4). The SYNTAX algorithm consists of twelve questions (Table 11.2). Rates of major adverse cardiac or cerebrovascular events at 12 months were significantly higher in the PCI group (17.8 %, vs. 12.4 % for CABG; P = 0.002), in large part because of an increased rate of repeat revascularization (13.5 % vs. 5.9 %, P < 0.001). At 12 months, the rates of death and myocardial infarction were similar between the two groups; stroke was significantly more likely to occur with CABG (2.2 %, vs. 0.6 % with PCI; P = 0.003). Results of SINTAX trial at 5 years of follow up (Fig. 11.5) showed Kaplan-Meier estimates of MACCE rates to be 26.9 % for the CABG group versus 37.3 % in the PCI group (p < 0.0001). The rates of myocardial infarction, the combination of death or stroke or myocardial infarction, and repeat revascularization were significantly higher in patients who were assigned to PCI than in those who were assigned to CABG. The rates of all-cause mortality and stroke, however, were not significantly different between groups. 28.6 % of patients in the CABG group with low SYNTAX scores had MACCE versus 32.1 % of patients in the PCI group (p = 0.43) and 31 % in the CABG group with left main coronary disease had MACCE versus 36.9 % in the PCI group (p = 0.12); however, in patients with intermediate or high SYNTAX scores, MACCE was significantly increased with PCI (intermediate score, 25.8 % of the CABG group vs 36.0 % of the PCI group; p = 0.008; high score, 26.8 % vs 44 %; p < 0.0001). The authors concluded that CABG should remain the standard of care for patients with high or intermediate SYNTAX scores. For patients with less complex disease (low SYNTAX scores) or left main coronary disease (low or intermediate SYNTAX scores), PCI was an acceptable alternative. One of the main implications derived from SYNTAX trial was the usefulness of the SYNTAX score, as a measure of the anatomical severity of coronary artery disease, and the assessment of the Heart Team in the revascularization decision-making process [34].
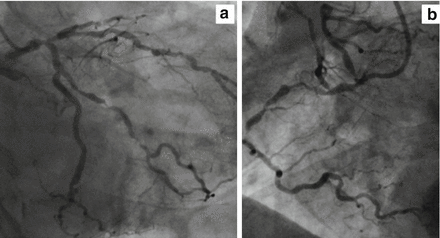
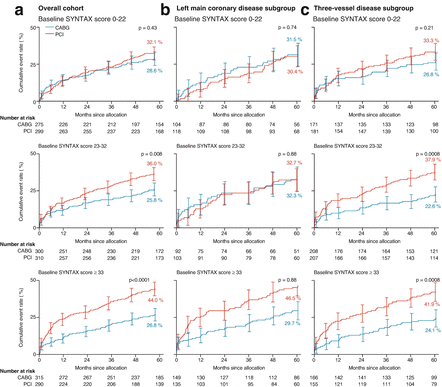

Figure 11.4
Angiographic view of a high SINTAX score case (>33). (a) Left coronary artery. (b) Right coronary artery
Table 11.2
The SYNTAX score algorithm
1. Dominance |
2. Number of lesions |
3. Segments involved per lesion |
4. Total occlusion |
(a) Number of segments involved |
(b) Age of the total occlusion (>3 months) |
(c) Blunt Stump |
(d) Bridging collaterals |
(e) First segment beyond the occlusion visible by antegrade or retrograde filling |
(f) Side branch involvement |
5. Trifurcation |
6. Bifurcation |
(a) Type |
(b) Angulation between the distal main vessel and the side branch <70° |
7. Aorto-ostial lesion |
8. Severe tortuosity |
9. Length >20 mm |
10. Heavy calcification |
11. Thrombus |
12. Diffuse disease/small vessels |

Figure 11.5
Kaplan-Meier cumulative event curves for MACCE by baseline SYNTAX score tercile. (a) Overall cohort; (b) left main coronary disease subgroup; and (c) three-vessel disease subgroup (Reproduced with permission from Mohr et al. [34])
In the left main setting, we have now the results of some studies comparing PCI and CABG. The PRECOMBAT trial was a prospective, open label, randomized trial conducted at 13 sites in Korea. The authors randomly assigned patients with unprotected left main coronary artery stenosis to CABG (300 patients) or PCI with sirolimus-eluting stents (300 patients). Both groups were compared with respect to the primary composite end point of major adverse cardiac or cerebrovascular events (death from any cause, myocardial infarction, stroke, or ischemia-driven target-vessel revascularization) at 1 year. Event rates at 2 years were also reported. PCI with sirolimus-eluting stents was shown to be noninferior to CABG with respect to the primary composite end point [35]. Results from DELTA registry showed that PCI for ostial/midshaft lesions in unprotected left main coronary artery was associated with clinical outcomes comparable to those observed with CABG at long-term follow-up, despite the use of older first-generation DES [36]. PCI for ostial/mid-shaft lesions has been also associated with better clinical outcomes compared to distal bifurcation lesions in unprotected left main coronary artery, due to the lower need for repeat revascularization in ostial/mid-shaft lesions [37]. Regarding the type of stent, no differences have been recently reported between the use of zotarolimus versus everolimus eluting stents in the left main PCI [38].
Revascularization therapy should be carefully assessed in an individualized fashion by the “Heart Team”, a group of different physicians involving clinical cardiologists, interventional cardiologists, and cardiac surgeons not directly implicated in the clinical case, who should report a global evaluation of each case. Many different factors should be taken in account to reach a decision regarding revascularization. The anatomical complexity of coronary artery disease (SYNTAX score), biological characteristics of the patient (i.e. age, diabetes, cerebrovascular disease, renal failure), surgical risk estimated by validated scores, the possibility of therapeutic fulfilment and the preference of the patient must be determinant factors.
Effect of Revascularization on Left Ventricular Function
Complete revascularization is considered to be one of the strongest predictors of improved clinical outcome [39–47]. While reduced left ventricular ejection fraction (LVEF) is known to be a powerful prognostic predictor of adverse clinical outcome, it remains unclear whether patients with SCAD and preserved left ventricular function who receive revascularization are more likely to preserve left ventricular systolic function as compared with those who receive OMT alone [39]. The results of several prospective randomized trials, which rigorously compared ‘hard’ clinical endpoints i.e. death and MI in SIHD patients who had undergone revascularization or OMT have failed to show superiority of either management strategy. In the Bypass Angioplasty Revascularization Investigation (BARI) trial, the evaluation of left ventricular function (initially preserved) was performed in 1,220 subjects with multivessel coronary heart disease. After 5 years from randomization (PTCA or CABG) there were no significant differences in left ventricular ejection fraction between groups [48]. In MASS II (Multivessel disease randomized for CABG, PCI and medical treatment) there were no differences in the evolution of left ventricular function between patients in the medical treatment group and those who underwent surgical or percutaneous revascularization [31]. Similar findings were reported in a sub analysis of COURAGE trial. The COURAGE nuclear sub study assessed ventricular function at baseline and 6–18 months after intervention. Similar rest and post-stress LVEF values were found in OMT treated patients, with or without PCI [49].
In a recently published post-hoc analysis of the MASS II Trial, Garzillo et al. [50] evaluated long-term findings of serial left ventricular ejection fraction measurements in stable ischemic heart disease patients with multivessel CAD at baseline and at 10 years following randomization to PCI, CABG, or OMT using transthoracic echocardiography. The principal finding of this post-hoc analysis [50] was that regardless of the therapeutic strategy applied, left ventricular function remained preserved in the absence of major adverse cardiac events, except for an obvious decrease in LVEF among the subset of SCAD patients who sustained an MI either prior to or after revascularization (Fig. 11.6). LV systolic function remained preserved at the end of the 10-year follow-up period with each of the three treatment strategies and declined minimally in all three groups. In conclusion, there was no statistically significant difference in the decline in LVEF between OMT and revascularization in this stable ischemic heart disease population. These data suggest that, in patients with multivessel stable coronary artery disease and preserved left ventricular systolic function at baseline, there is no measurable difference in left ventricular ejection fraction with or without revascularization [50]. Further studies are necessary for a deeper knowledge about the real influence of complete or incomplete revascularization in SCAD patients for preservation of left ventricular function.
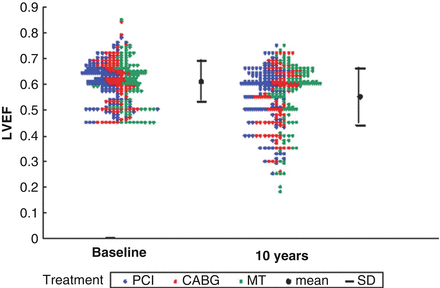

Figure 11.6
Left ventricular ejection fraction at baseline and the 10th year, according to treatment assigned: p = 0.675: mean left ventricular ejection fraction similar among groups in the beginning and end of the follow-up; p = 0.641: similar evolution among the groups; p = 0.001: left ventricular ejection fraction decrease over time (Reproduced with permission from Garzillo et al. [50])
Ischemia-Guided Percutaneous Revascularization
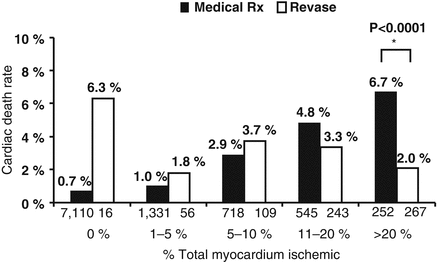
Revascularization therapy for SCAD guided by tests of ischaemia is one of the principal hot topics of the actual guidelines. The presence and extent of inducible ischemia is one of the most important factors related to outcome [51]. In patients with SCAD, several studies have analyzed the role of ischemia-guided percutaneous revascularization, using non invasive and invasive methods. Non invasive imaging studies with exercise echocardiography suggest that treatment of non ischemic coronary lesions may be safely deferred with an annual estimated incidence of death or MI of 0.6 %, as reported in the meta-analysis of Meltz et al [52]. The study of Hachamovitch et al. [53] emphasized the relationship between inducible ischemia on SPECT and the presence of short-term survival benefits with early revascularization vs. medical therapy. 10,627 patients without a prior history of MI or revascularization were included and underwent SPECT imaging. Revascularization was associated with a reduction in mortality for patients having moderate to severe ischemia. The cut-off level of ischemic myocardium, assessed by summed stress scores, to predict lower mortality using revascularization was approximately 10–12.5 % (Fig. 11.7) [53]. Kim et al. supported the benefit of ischemia-guided revascularization with myocardial perfusion imaging for patients with multivessel coronary artery disease. The outcomes of ischemia-guided revascularization were retrospectively compared with those of non ischemia-guided revascularization in a registry of 5,340 patients with multivessel coronary disease comprising 2,587 percutaneous coronary interventions (PCIs) with drug-eluting stents and 2,753 coronary artery bypass graft (CABG) surgeries. The incidence of major adverse cardiac and cerebrovascular events (MACCE) including death, myocardial infarction, stroke, or repeat revascularization was significantly lower in the ischemia-guided than in the non ischemia-guided group (16.2 % vs. 20.7 %; adjusted hazard ratio [aHR]: 0.73; 95 % confidence interval [CI]: 0.60–0.88; p = 0.001), primarily driven by the lower repeat revascularization rate (9.9 % vs. 22.8 %; aHR: 0.66; 95 % CI: 0.49–0.90; p = 0.009). Subgroup analysis showed that ischemia-guided reduced the risk of MACCE in PCI (17.4 % vs. 22.8 %; aHR: 0.59; 95 % CI: 0.43–0.81; p = 0.001) but not in CABG (16.0 % vs. 18.5 %; aHR: 0.87; 95 % CI: 0.67–1.14; p = 0.31) patients [54]. Regarding diagnostic procedures, several diagnostic modalities are available for use as tools to establish the initial diagnosis, assess disease severity, and select the appropriate treatment strategy in symptomatic patients suspected of having CAD. In relation to this point, a multicenter study performed in Japan hypothesised that the choice of the initial diagnostic test might influence the treatment strategy. They showed that patients receiving initial SPECT had a lower rate of revascularization than those receiving coronary angiography (odds ratio, 5.36; 95 % CI, 4.07–7.05) [55].