INTRODUCTION
Mycology is the study of fungi, which are eukaryotic organisms that evolved in tandem with the animal kingdom. However, unlike animals, most fungi are nonmotile and possess a rigid cell wall. Unlike plants, fungi are nonphotosynthetic. Approximately 80,000 species of fungi have been described, but only about 400 are medically important, and less than 50 are responsible for more than 90% of the fungal infections of humans and other animals. Rather, most species of fungi are beneficial to humankind. They reside in nature and are essential in breaking down and recycling organic matter. Some fungi greatly enhance our quality of life by contributing to the production of food and spirits, including cheese, bread, and beer. Other fungi have served medicine by providing useful bioactive secondary metabolites such as antibiotics (eg, penicillin) and immunosuppressive drugs (eg, cyclosporine). Fungi have also been exploited by geneticists and molecular biologists as model systems for the investigation of a variety of eukaryotic processes, including cellular growth and development. Overall, fungi exert their greatest economic impact as phytopathogens; the agricultural industry sustains huge crop losses every year as a result of fungal diseases of rice, corn, grains, and other plants.
Like all eukaryotes, each fungal cell has at least one nucleus with a nuclear membrane, endoplasmic reticulum, mitochondria, and secretory apparatus. Most fungi are obligate or facultative aerobes. They are chemotrophic, secreting enzymes that degrade a wide variety of organic substrates into soluble nutrients which are then passively absorbed or taken into the cell by active transport.
The term mycoses refers to infections that are caused by fungi. Most pathogenic fungi are exogenous, their natural habitats being water, soil, and organic debris. The mycoses with the highest incidence—candidiasis and dermatophytosis—are caused by fungi that are frequent components of the normal human microbiota and highly adapted to survival on the human host. For convenience, mycoses may be classified as superficial, cutaneous, subcutaneous, or systemic, invading the internal organs (Table 45-1). The systemic mycoses may be caused by endemic fungi, which are usually primary pathogens and geographically restricted, or by ubiquitous, often secondary opportunistic pathogens. Grouping mycoses in these categories reflects their most common portal of entry and initial site of involvement. However, there is considerable overlap, since systemic mycoses often exhibit subcutaneous manifestations and vice versa. Most patients who develop opportunistic infections have serious underlying diseases and compromised host defenses. But primary systemic mycoses also occur in such patients, and the opportunists often infect immunocompetent individuals.
| Category | Mycosis | Causative Fungal Agents |
|---|---|---|
| Superficial | Pityriasis versicolor Tinea nigra White piedra Black piedra | Malassezia species Hortaea werneckii Trichosporon species Piedraia hortae |
| Cutaneous | Dermatophytosis Candidiasis of skin, mucosa, or nails | Microsporum species, Trichophyton species, and Epidermophyton floccosum Candida albicans and other Candida species |
| Subcutaneous | Sporotrichosis Chromoblastomycosis Mycetoma Phaeohyphomycosis | Sporothrix schenckii Phialophora verrucosa, Fonsecaea pedrosoi, and others Pseudallescheria boydii, Madurella mycetomatis, and others Exophiala, Bipolaris, Exserohilum, and other dematiaceous molds |
| Endemic (primary, systemic) | Coccidioidomycosis Histoplasmosis Blastomycosis Paracoccidioidomycosis | Coccidioides posadasii and Coccidioides immitis Histoplasma capsulatum Blastomyces dermatitidis Paracoccidioides brasiliensis |
| Opportunistic | Systemic candidiasis Cryptococcosis Aspergillosis Hyalohyphomycosis Phaeohyphomycosis Mucormycosis (zygomycosis) Pneumocystis pneumonia Penicilliosis | Candida albicans and many other Candida species Cryptococcus neoformans and Cryptococcus gattii Aspergillus fumigatus and other Aspergillus species Species of Fusarium, Paecilomyces, Trichosporon, and other hyaline molds Cladophialophora bantiana; species of Alternaria, Cladosporium, Bipolaris, Exserohilum, and numerous other dematiaceous molds Species of Rhizopus, Lichtheimia, Cunninghamella, and other members of the Order Mucorales Pneumocystis jiroveci Penicillium marneffei |
GLOSSARY
Budding: A common mode of asexual reproduction, typical of yeasts. During mitosis, the parent cell wall protrudes outwardly and enlarges to form a nascent bud that contains the progeny nucleus. A fungal cell may produce single or multiple buds.
Conidia: Asexual reproductive structures (mitospores) produced either from the transformation of a vegetative yeast or hyphal cell or from a specialized conidiogenous cell, which may be simple or complex and elaborate. Conidia may be formed on specialized hyphae, termed conidiophores. Microconidia are small, and macroconidia are large or multicellular.
Arthroconidia (arthrospores): Conidia that result from the fragmentation of hyphal cells (Figure 45-1).
Blastoconidia (blastospores): Conidia that are produced by budding (eg, yeasts).
Chlamydospores (chlamydoconidia): Large, thick-walled, usually spherical conidia produced from terminal or intercalary hyphal cells.
Phialoconidia: Conidia that are produced by a “vase-shaped” conidiogenous cell termed a phialide (eg, Aspergillus fumigatus, Figure 45-6).
Dematiaceous fungi: Fungi whose cell walls contain melanin, which imparts a brown to black pigment.
Dimorphic fungi: Fungi that have two growth forms, such as a mold and a yeast, which develop under different growth conditions (eg, Blastomyces dermatitidis forms hyphae in vitro and yeasts in tissue).
Hyphae: Tubular, branching filaments (2–10 μm in width) of fungal cells, the mold form of growth. Most hyphal cells are separated by porous cross-walls or septa, but in the Order Mucorales, the hyphae are characteristically sparsely septate. Vegetative or substrate hyphae anchor the colony and absorb nutrients. Aerial hyphae project above the colony and bear the reproductive structures.
Anamorph: The mitotic or asexual reproductive state of a fungus. Anamorphic fungal taxa are identified on the basis of their asexual reproductive structures (ie, mitospores).
Mold: Hyphal or mycelial colony or form of growth.
Mycelium: Mass or mat of hyphae, mold colony.
Teleomorph: The sexual reproductive state of a fungus, which involves plasmogamy, karyogamy, and meiosis.
Pseudohyphae: Chains of elongated buds or blastoconi-dia; the septations between cells are constricted.
Septum: Hyphal cross-wall, typically perforated.
Sporangiospores: Asexual structures characteristic of the Order Mucorales; they are mitotic spores produced within an enclosed sporangium, often supported by one sporangiophore (see Figures 45-2 and 45-3).
Spore: A specialized propagule with enhanced survival value, such as resistance to adverse conditions or structural features that promote dispersion. Spores may result from asexual (eg, conidia, sporangiospores) or sexual (see below) reproduction.
Sexual spores: During sexual reproduction, haploid cells of compatible strains mate through a process of plasmogamy, karyogamy, and meiosis.
Ascospores: In the Phylum Ascomycota, following meiosis, four to eight meiospores form within an ascus.
Basidiospores: In the Phylum Basidiomycota, following meiosis, four meiospores usually form on the surface of a specialized structure, a club-shaped basidium.
Zygospores: In the Order Mucorales, following meiosis, a large, thick-walled zygospore develops.
Yeasts: Unicellular, spherical to ellipsoid (3–15 μm) fungal cells that usually reproduce by budding.
During a fungal infection, most patients develop significant cellular and humoral immune responses to fungal antigens. Much of the continuing increase in opportunistic mycoses can be attributed to medical advances that have significantly prolonged the survival of patients with cancer, AIDS, and hematopoietic stem cell or solid organ transplants. As these clinical data suggest, Th1 and Th17 immune responses are critical host defense mechanisms for natural protection against life-threatening mycoses. Pathogenic fungi do not produce potent toxins, and the features of fungal pathogenicity are complex and polygenic. In addition, experimental infections have shown that populations of a pathogenic species vary in their virulence, and genetic studies have identified numerous genes that contribute to fungal pathogenicity.
Most mycoses are difficult to treat. Because fungi are eukaryotes, they share numerous homologous genes, gene products, and pathways with their human hosts. Consequently, there are few unique targets for chemotherapy. However, there is growing interest in the search for potential therapeutic targets and new antifungal drugs are becoming available.
GENERAL PROPERTIES, VIRULENCE, AND CLASSIFICATION OF PATHOGENIC FUNGI
Fungi have two basic growth forms, as molds and yeasts. Growth in the mold (or mould) form occurs by the production of multicellular branching cylindrical tubules called hyphae that vary in diameter from 2 to 10 μm. Hyphae are extended by apical elongation due to the production of new cell wall growth at the hyphal tips. The mass of intertwined hyphae that accumulates during active growth is a mycelium. Some hyphae are divided into cells by cross-walls or septa, which typically form at regular intervals during hyphal growth. However, members of the Order Mucorales produce hyphae that are rarely septated. Vegetative or substrate hyphae penetrate the supporting medium, anchor the colony, and absorb nutrients. In contrast, aerial hyphae project above the surface of the mycelium and usually bear the reproductive structures of the mold. When a mold is isolated from a clinical specimen, its growth rate, macroscopic appearance, and microscopic morphology are usually sufficient to determine its genus and species. The most helpful phenotypic features are the ontogeny and morphology of the asexual reproductive spores, or conidia (Figures 45-2, 45-3, 45-4, 45-5, 45-6, 45-7, and 45-8).
Yeasts are single cells, usually spherical to ellipsoid in shape and varying in diameter from 3 to 15 μm. Most yeasts reproduce by budding, which is initiated by a lateral or terminal protrusion of new cell wall growth that enlarges during mitosis. One or more replicated nuclei enter the nascent bud, which subsequently forms a septum and separates from the parent cell. Some species produce buds that characteristically fail to detach and become elongated; this continuation of the budding process produces chains of elongated yeast cells called pseudohyphae. Yeast colonies are usually soft, opaque, 1–3 mm in size, and cream colored. The colonies and microscopic morphology of many species of yeasts appear quite similar, but they can be identified by physiologic tests and a few key morphologic differences. Some species, including several pathogens, are dimorphic and capable of growth as a yeast or mold depending on environmental conditions, such as temperature or available nutrients.
The life cycles of fungi are remarkably versatile. Depending on the fungus, the predominant nuclear chromosomal count may be haploid or diploid. Some species exist entirely by clonal growth or asexual reproduction, and barring spontaneous mutations, every cell will be a genetic clone. Many other species are capable of sexual reproduction, which may or may not require genetically different partners for mating and meiosis. Asexual as well as sexual reproduction can result in the production of spores, which enhance fungal survival. Spores are usually dormant, readily dispersed, more resistant to adverse conditions, and germinate to form vegetative cells when conditions for growth are favorable. Spores derived from asexual or sexual reproduction are termed anamorphic or teleomorphic, respectively. Like vegetative cells, asexual spores are mitotic progeny (ie, mitospores). The medical fungi produce two major types of asexual spores, conidia, which are produced by most pathogenic fungi, and, in the Order Mucorales, sporangiospores (see below and Glossary). Informative features of spores include their ontogeny (some molds produce complex conidiogenic structures) as well as their morphology (size, shape, texture, color, and unicellularity or multicellularity). In some fungi, vegetative cells may transform into conidia (eg, arthroconidia, chlamydospores). In others, conidia are produced by a conidiogenous cell, such as a phialide, which itself may be attached to a specialized hypha called a conidiophore. Sporangiospores result from mitotic replication and spore production within a sac-like structure called a sporangium, which is supported by a sporangiophore.
Certain fungal properties are essential but not necessarily sufficient for pathogenicity, such as the ability to proliferate in the mammalian host. Many virulence factors have evolved to enable pathogenic fungi to withstand or circumvent the defenses and stressful environment of the host. Some of these virulence determinants include morphological transformations, genetic “switching” of metabolic processes in response to the host environment, the production of surface adhesins that bind to host cell membranes, the secretion of enzymes that attack host substrates (eg, catalase, aspartyl proteinases, phospholipases), cell wall components that resist phagocytosis (eg, α-(1,3)-glucan, melanin, the capsule of Cryptococcus), and the formation of biofilms. Specific examples are provided in this chapter’s descriptions of several mycoses.
Fungi have an essential rigid cell wall that determines their shape and protects them from osmotic and other environmental stresses. Cell walls are composed largely of carbohydrate layers—long chains of polysaccharides—as well as glycoproteins and lipids. Some sugar polymers are found in the cell walls of many fungi, such as chitin (an unbranched polymer of β-1,4-linked N-acetylglucosamine); glucans, which are glucose polymers (eg, α-1,3-glucan, β-1,3-glucan, and β-1,6-glucan); and mannans, polymers of mannose (eg, α-1,6-mannose). These components are cross-linked to from a multilayered cell wall matrix. In addition, other polysaccharides may be unique to specific fungal species and therefore useful for identification. During infection, fungal cell walls exert important pathobiologic properties. The surface components of the cell wall mediate attachment of the fungus to host cells. Specific fungal cell wall moieties bind to pattern recognition receptors on host cell membranes, such as certain Toll-like receptors, to stimulate innate immune responses. Cell wall glucans and other polysaccharides may activate the complement cascade and provoke an inflammatory reaction. Most of these polysaccharides are poorly degraded by the host and can be detected with special histologic stains. Cell walls also release immunodominant antigens that may elicit cellular immune responses and diagnostic antibodies. In addition, some yeasts and molds are described as dematiaceous because their cell walls contain melanin, which imparts a brown or black pigment to the fungal colony. Several studies have shown that melanin protects these fungi from host defenses.
Fungi were initially classified into phyla based largely on their modes of sexual reproduction and phenotypic data. These methods have been supplanted by molecular systematics, which more accurately reflect phylogenetic relationships. There is some ambiguity about the divergence of fungi and animals and their extant ancestors. The lower fungi were assigned to the Phylum Zygomycota, but this phylum was shown to be polyphyletic and has been replaced by the Phylum Glomerulomycota, four subphyla and two zoopathogenic Orders, the Mucorales and the Entomophthorales. However, the two largest phyla, Ascomycota and Basidiomycota, are well supported by phylogenetic analyses. All three of these phyla contain yeasts, molds, and dimorphic species. The Phylum Ascomycota (or ascomycetes) includes more than 60% of the known fungi and about 85% of the human pathogens. Most of the other pathogenic fungi are members of the Phylum Basidiomycota (basidiomycetes) or the Order Mucorales. These medically relevant taxa are distinguished by their modes of reproduction. Sexual reproduction typically occurs when mating-compatible strains of a species are stimulated by pheromones to undergo plasmogamy, karyogamy (nuclear fusion), and meiosis, resulting in the exchange of genetic information and the formation of haploid sexual spores.
For molds within the Order Mucorales, the vegetative hyphae have few septations, the product of sexual reproduction between mating-compatible isolates is a zygospore, and asexual reproduction occurs via sporangia, which are borne on aerial sporangiophores (see Glossary). Examples include species of Rhizopus, Lichtheimia, and Cunninghamella. Among the ascomycetes, sexual reproduction usually requires the fusion of mating-compatible strains and involves the formation of a sac or ascus in which karyogamy and meiosis occur, producing haploid ascospores. They reproduce asexually with the production of conidia. Ascomycetous molds have septate hyphae. Most pathogenic yeasts (Saccharomyces, Candida) and molds (Coccidioides, Blastomyces, Trichophyton) are ascomycetes. The basidiomycetes include mushrooms as well as pathogenic species of Cryptococcus. Sexual reproduction results in dikaryotic hyphae and four progeny basidiospores that are supported by a club-shaped basidium. In the diagnostic laboratory, multiple approaches are deployed to identify clinical isolates, including molecular and phenotypic features (eg, signature DNA sequences, morphology of reproductive structures, physiologic properties). Clinical isolates almost always represent infection by a single clone and reproduce asexually in the laboratory. Consequently, many pathogens were initially classified according to their asexual reproductive structures or anamorphic states, and with the subsequent discovery of a sexual cycle, such taxa acquired a teleomorphic name. During the evolution to become successful pathogens, some fungi have apparently lost the ability to reproduce sexually.
LABORATORY DIAGNOSIS OF MYCOSES
The vast majority of fungi have evolved to reside in various environmental niches where they grow readily on vicinal organic substrates and are protected from deleterious conditions. Although these exogenous fungi are unable to penetrate the intact surfaces of healthy hosts, they may be acquired accidentally by traumatic exposure to resident fungi in soil, water, air, or vegetation. Once fungal cells have breached the cutaneous or mucosal surfaces, such as the skin, or the respiratory, urinary, or gastrointestinal tract, they are repelled by innate host defenses. Potentially pathogenic fungi must be able to grow at 37°C, acquire essential nutrients from the host, and evade the immune responses. The few hundred environmental fungi with these attributes represent only a tiny percentage of global species. Unfortunately, a few highly prevalent fungi with these abilities are able to cause opportunistic, invasive infections in patients with compromised host defenses (eg, aspergillosis, cryptococcosis). Overall, most mycoses are caused by noninvasive molds that have adapted to grow on the skin, hair, or nails, and by endogenous species of Candida, which are members of the human mycobiome. However, regardless of their source, with the exception of dermatophytes, pathogenic fungi are not contagious, and transmission among humans or animals is extremely rare. This chapter describes the most prevalent mycoses, but new pathogens are reported every year. There is also brief coverage of two different mechanisms whereby fungi may cause human disease—ingestion of fungal toxins or exposure to fungal cell wall components that elicit IgE-mediated allergic responses.
In general, the most definitive methods to establish the diagnosis of a fungal infection are culture of the pathogen, microscopic examination, detection of species-specific fungal DNA, and serology. Since these methods vary in their availability, specificity, sensitivity, methods, and turnaround time, it is prudent to utilize several diagnostic strategies.
Clinical specimens collected for microscopy and culture are determined by the site(s) of infection and the condition of the patient. All specimens should be obtained using aseptic technique, especially with specimens from normally sterile sites, such as blood, tissue biopsies, cerebrospinal fluid, and so forth. Specimens from nonsterile body sites include skin and subcutaneous lesions, nasopharyngeal or genital swabs, bronchoalveolar lavage fluid, urine, and wounds. To minimize bacterial growth, specimens should be transported to the diagnostic laboratory within two hours. Whenever a fungal infection is suspected, alert the diagnostic laboratory because special stains and culture media have been developed for the detection of pathogenic fungi.
One or two drops of an aqueous or serous specimen, such as sputum, urine, spinal fluid, or aspirate, can be placed on a glass slide in a drop of 10–20% potassium hydroxide (KOH), and after adding a coverslip, the slide is examined under the microscope with the low- and high-power (450×) objectives. KOH dissolves any tissue cells, and the resistant, highly refractory fungal cell walls become more visible. This procedure can also be used to examine skin scrapings or minced tissue samples. The sensitivity of the KOH solution is improved by the addition of calcofluor white, which is a nonspecific fungal cell wall stain that is visible with a fluorescent microscope. The detection of fungi in pus, viscous exudates, and minced tissue can also be examined with KOH preps by gently heating the slide to dissolve excess tissue debris and inflammatory cells. Fungi can also be observed in blood smears, CSF, and other preps treated with Gram or Wright stain.
In formalin-fixed biopsy specimens, fungi can be detected with the routine hematoxylin and eosin (H&E) histopathological stain. However, specialized fungal cell wall stains are more sensitive. The two most common stains are Gomori-methenamine silver (GMS) and periodic acid-Schiff (PAS), which stain fungal walls black or red, respectively. Other specialized stains, such as capsule stains for Cryptococcus, are described in subsequent sections.
Although the sensitivity of microscopic examinations varies with the mycosis and the extent of disease, this examination can be performed very quickly and is often definitive. In the host, most fungi grow as yeasts, hyphae, or a combination of yeasts and pseudohyphae. Table 45-2 lists the spectrum of in vivo fungal structures. In many cases, the pathogens that are present only as yeasts are sufficiently distinctive in size and shape to establish an immediate diagnosis. In other cases, based on fungal appearance (eg, nonseptate hyphae or brownish cell walls) and the specimen site (eg, superficial or systemic), the list of possible fungal agents is considerably narrowed.
| Predominant Morphology | Mycoses |
|---|---|
| Yeasts—single or multiple buds | Blastomycosis, Histoplasmosis, Paracoccidioidomycosis, Penicilliosis, Sporotrichosis |
| Yeasts with capsules | Cryptococcosis |
| Hyphae—septate | Hyalohyphomycosis—species of Aspergillus, Fusarium, Geotrichum, Trichosporon, et al.) |
| Hyphae—septate in skin or nail specimens | Dermatophytosis |
| Hyphae—nonseptate | Mucormycosis—species of Rhizopus, Lichtheimia, Cunninghamella, et al. |
| Hyphae—septate; brownish cell walls | Phaeohyphomycosis—species of Bipolaris, Cladosporium, Curvularia, Exserohilum, et al. |
| Yeasts and Pseudohyphae | Candidiasis—species of Candida |
| Yeasts and Hyphae in skin scrapings | Pityriasis versicolor |
| Spherules | Coccidioidomycosis |
| Sclerotic cells—brownish cell walls | Chromoblastomycosis |
| Sulfur granules | Mycetoma |
| Arthroconidia in hair | Dermatophytosis |
| Conidia in pulmonary cavity | Hyalohyphomycosis—species of Aspergillus, Fusarium, et al. |
| Cysts (asci) in pulmonary specimens | Pneumocystis |
In most cases, the culture is more sensitive than the direct examination, and a portion of the material collected for microscopy should be cultured. The traditional mycological medium, Sabouraud’s dextrose agar (SDA), contains glucose and modified peptone (pH 7.0), supports the growth of fungi, and restricts the growth of bacteria. The morphologic characteristics of fungi used for identification have been described from growth on SDA. However, other media, such as inhibitory mold agar (IMA), enhance the recovery of fungi from clinical specimens. To culture medical fungi from nonsterile specimens, antibacterial antibiotics (eg, gentamicin, chloramphenicol) and cycloheximide are added to the media to inhibit bacteria and saprobic molds, respectively. After cultures are obtained, Potato Dextrose Agar stimulates the production of conidia.
For culturing blood, several commercial broth media have been developed for bacteria and/or fungi. Most yeast species in blood can be detected and subcultured from these media within three days. However, molds may require several weeks of incubation to become positive, and hence, special procedures must be used to optimize their recovery. Yeasts grow better at 37°C, and molds at 30°C. When a dimorphic fungus is suspected, multiple media and incubation temperatures are recommended. The ideal method for likely cases of fungemia is a commercial lysis-centrifugation (Isolator®) tube to which blood is directly added. The tube contains an anticoagulant and detergent to lyse the blood cells, releasing any phagocytized fungal cells, and the tube is then centrifuged to pellet any fungi. After decanting the supernatant fluid, the pellet is suspended, streaked on agar plates of mycologic media, and incubated. Positive cultures of most yeasts or molds can be identified by morphological and physiological phenotypes. Several commercial microculture systems for yeasts are able to generate substrate assimilation profiles, and these profiles can be compared with large databases to identify most pathogenic species of yeasts. As described later, specialized media are available to assist in the rapid identification of Candida species (eg, CHROMagar®).
The following sections of this chapter will explain how the detection of specific antibodies or antigens in serum or CSF can provide useful diagnostic and/or prognostic information. In immunocompetent patients, positive antibody tests may confirm the diagnosis, and negative tests may exclude fungal disease. However, the interpretation of each serologic test depends upon its sensitivity, specificity, and predictive value in the patient population being tested.
An increasing number of clinical laboratories have implemented methods based on the detection of fungal nucleic acids, proteins, or antigens to identify pathogenic fungi in clinical specimens or after their recovery in culture. Multiple approaches have been published, and in-house as well as commercial systems are available, but none have been widely adopted. In the next decade one or more of these methods may become routine, especially if they can be automated, provide rapid throughput, and detect multiple microbes. In addition, some platforms have the potential for point-of-care usage. Most DNA-based methods use PCR to amplify fungus-specific sequences of ribosomal DNA or other conserved genes. By comparing the DNA sequences of a clinical isolate with databases of thousands of fungal DNA sequences, the genus or species of an unknown fungus can be identified. This approach has been used to identify cultures of hundreds of fungal taxa. In addition, a variety of reports have used PCR to detect fungal DNA (mostly Candida or Aspergillus) in blood and other specimens.
Automated commercial instruments for the DNA-based identification of pathogenic fungi have been developed by several companies, including Luminex Molecular Diagnostics®, Gen-Probe®, LightCycler® SeptiFast, and MicroSeq®. For detection, they typically use oligonucleotide probes that emit a signal amplified by fluorescence, chemical reagents, or enzyme immunoassay. For detecting fungal cells in slide preparations, a PNA-FISH® (peptide-nucleic acid fluorescent in situ hybridization) test kit with species-specific probes can be used.
DNA-based fingerprinting methods, such as multilocus sequence typing (MLST), have identified phylogenetic subpopulations of many pathogenic fungi, including species of Candida, Cryptococcus, Aspergillus, and Coccidioides. Some of these molecular subgroups are clinically relevant as they are associated with differences in geographic distribution, susceptibility to antifungal drugs, clinical manifestations, or virulence. Some of these subgroups may represent cryptic species that cannot be differentiated by phenotypic methods.
Another molecular method that is gaining currency because of its application to pathogenic bacteria as well as fungi involves the extraction of microbial proteins, which are then submitted to mass spectroscopy. Pathogens are identified by comparing their protein spectral patterns with those in databases of previously tested species. With the ease of sample preparation and the commercial availability of automated instruments, this method—matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS)—is becoming more accessible. In several studies, MALDI-TOF-MS has proven to be more accurate and faster than conventional culture methods. Most of the current data are based on the identification of species of Candida.
Similar to the rapid test for endotoxin, the clotting cascade of the horseshoe crab (Limulus) hemolymph is also triggered by fungal cell wall β-(1,3)-d-glucan. This reaction (Fungicell®) has been exploited to quantify its concentration in blood and spinal fluid. β-(1,3)-d-glucan levels of ≥80 pg/mL are positive and associated with invasive candidiasis, aspergillosis, dimorphic pathogens, and other mycoses.
SUPERFICIAL MYCOSES
Pityriasis versicolor is a highly prevalent, chronic superficial infection of the stratum corneum caused by species of the lipophilic yeast, Malassezia. These yeasts can be isolated from normal skin and scalp and are considered part of the cutaneous mycobiota. Thus, infections are likely caused by endogenous strains. There are 14 currently recognized species of Malassezia, but the vast majority of cases of pityriasis versicolor are caused by Malassezia globosa, Malassezia furfur, or Malassezia sympodialis. The infection is characterized by discrete, serpentine, hyper-, or hypopigmented maculae that develop on the skin, usually on the chest, upper back, arms, or abdomen. These patches of discolored skin may enlarge and coalesce, but scaling, inflammation, and irritation are minimal. Indeed, this common affliction is largely a cosmetic problem. Pityriasis versicolor affects all ages, and the annual incidence is reportedly 5–8%. Predisposing conditions include the immune status of the patient, genetic factors, and elevated temperature and humidity.
Most species of Malassezia require lipid in the medium for growth. The diagnosis is confirmed by direct KOH microscopic examination of scrapings of infected skin. Short unbranched, nonpigmented hyphae and spherical cells are observed. The lesions also fluoresce under Wood’s lamp. Pityriasis versicolor is treated with daily applications of selenium sulfide. Topical or oral azoles are also effective. The goal of treatment is not to eradicate Malassezia from the skin but to reduce the cutaneous population to commensal levels.
The species of Malassezia noted above, as well as Malassezia restricta, have been implicated as causes or contributors to seborrheic dermatitis (ie, dandruff). This etiology is supported by the observation that many cases are alleviated by treatment with ketoconazole. Rarely, Malassezia may cause an opportunistic fungemia in patients—usually infants—receiving total parenteral nutrition (TPN), as a result of contamination of the lipid emulsion. In most cases, the fungemia is transient and eliminated by replacing the TPN and intravenous catheter. In addition, another less common manifestation of Malassezia is folliculitis.
Tinea nigra (or tinea nigra palmaris) is a superficial chronic and asymptomatic infection of the stratum corneum caused by the dematiaceous fungus Hortaea (Exophiala) werneckii. This condition is more prevalent in warm coastal regions and among young women. The lesions appear as a dark (brown to black) discoloration, often on the palm. Microscopic examination of skin scrapings from the periphery of the lesion will reveal branched, septate hyphae and budding yeast cells with melanized cell walls. Tinea nigra will respond to treatment with keratolytic solutions, salicylic acid, or azole antifungal drugs.
Black piedra is a nodular infection of the hair shaft caused by Piedraia hortae (Figure 45-9B). White piedra, due to infection with Trichosporon species, presents as larger, softer, yellowish nodules on the hairs (see Figure 45-9A). Hair of the axilla, genitalia, beard, and scalp hair may be infected. Treatment for both types consists of removal of the infected hair and application of a topical antifungal agent. Piedra is endemic in tropical countries.
CUTANEOUS MYCOSES
Cutaneous mycoses are caused by fungi that infect only the keratinized tissue (skin, hair, and nails). The most important of these are the dermatophytes, a group of about 40 related fungi that belong to three genera: Microsporum, Trichophyton, and Epidermophyton. Dermatophytes are restricted to the nonviable skin because most are unable to grow at 37°C or in the presence of serum. Dermatophytoses are among the most prevalent infections in the world. Although they can be persistent and troublesome, they are rarely debilitating or life-threatening—yet billions of dollars are expended annually in their treatment. Being superficial, dermatophyte (ringworm) infections have been recognized since antiquity. In skin they are diagnosed by the presence of hyaline, septate, branching hyphae, or chains of arthroconidia. In culture, the many species are closely related and often difficult to identify. They are speciated on the basis of subtle differences in the appearance of the colonies and microscopic morphology as well as a few vitamin requirements. Despite their similarities in morphology, nutritional requirements, surface antigens, and other features, many species have particular keratinases, elastases, and other enzymes that enable them to be quite host-specific. The identification of closely related and outbreak strains has been greatly aided by DNA sequence analysis. The several dermatophytic species that are capable of sexual reproduction produce ascospores and belong to the teleomorphic genus Arthroderma.
Dermatophytes are acquired by contact with contaminated soil or with infected animals or humans. The species are classified as geophilic, zoophilic, or anthropophilic depending on whether their usual habitat is soil, animals, or humans. Several dermatophytes that normally reside in soil or are associated with particular animal species are also able to cause human infections. In general, as a species evolves from habitation in soil to a specific animal or human host, it loses the ability to produce asexual conidia and to reproduce sexually. Anthropophilic species cause the greatest number of human infections. They elicit relatively mild and chronic infections, produce few conidia in culture, and may be difficult to eradicate. Conversely, geophilic and zoophilic dermatophytes, being less adapted to human hosts, produce more acute inflammatory infections that tend to resolve more quickly.
Some anthropophilic species are geographically restricted, but others, such as Epidermophyton floccosum, Trichophyton mentagrophytes var interdigitale, Trichophyton rubrum, and Trichophyton tonsurans, are globally distributed. The most common geophilic species causing human infections is Microsporum gypseum. Cosmopolitan zoophilic species (and their natural hosts) include Microsporum canis (dogs and cats), Microsporum gallinae (fowl), Microsporum nanum (pigs), Trichophyton equinum (horses), and Trichophyton verrucosum (cattle).
The more common dermatophytes are identified by their colonial appearance and microscopic morphology after growth for 2 weeks at 25°C on SDA. Trichophyton species, which may infect hair, skin, or nails, develop cylindric, smooth-walled macroconidia and characteristic microconidia (Figure 45-10A). Depending on the variety, colonies of T mentagrophytes may be cottony to granular; both types display abundant grape-like clusters of spherical microconidia on terminal branches. Coiled or spiral hyphae are commonly found in primary isolates. The typical colony of T rubrum has a white, cottony surface and a deep red, nondiffusible pigment when viewed from the reverse side of the colony. The microconidia are small and piriform (pear-shaped). T tonsurans produces a flat, powdery to velvety colony on the obverse surface that becomes reddish brown on reverse; the microconidia are mostly elongate (see Figure 45-10A).
FIGURE 45-10
Examples of the three genera of dermatophytes. A: Trichophyton tonsurans is characterized by the production of elongated microcondia attached to a supporting hypha. B: Microsporum gypseum produces individual thin- and rough-walled macroconidia. C: Epidermophyton floccosum has club-shaped, thin- and smooth-walled macroconidia that typically arise in small clusters.
Microsporum species tend to produce distinctive multicellular macroconidia with echinulate walls (see Figure 45-10B). Both types of conidia are borne singly in these genera. M canis forms a colony with a white cottony surface and a deep yellow color on reverse; the thick-walled, 8- to 15-celled macroconidia frequently have curved or hooked tips. M gypseum produces a tan, powdery colony and abundant thin-walled, four- to six-celled macroconidia. Microsporum species infect only hair and skin.
E floccosum, which is the only pathogen in this genus, produces only macroconidia, which are smooth-walled, clavate, two- to four-celled, and formed in small clusters (see Figure 45-10C). The colonies are usually flat and velvety with a tan to olive-green tinge. E floccosum infects the skin and nails but not the hair.
In addition to gross and microscopic morphology, a few nutritional or other tests, such as growth at 37°C or a test for in vitro hair perforation, are useful in differentiating certain species. Atypical isolates can usually be identified by species-specific polymerase chain reaction (PCR) tests.
Dermatophyte infections begin in the skin after trauma and contact. There is evidence that host susceptibility may be enhanced by moisture, warmth, specific skin chemistry, composition of sebum and perspiration, youth, heavy exposure, and genetic predisposition. The incidence is higher in hot, humid climates and under crowded living conditions. Shoes provide warmth and moisture, a setting for infections of the feet. The source of infection is soil or an infected animal in the case of geophilic and zoophilic dermatophytes, respectively. Anthropophilic species may be transmitted by direct contact or through fomites, such as contaminated towels, clothing, shared shower stalls, and similar examples. Unlike other fungal infections, dermatophytes are contagious and frequently transmitted by exposure to shed skin scales, nails, or hair containing hyphae or conidia. These fungal elements can remain viable for long periods on fomites.
Trichophytin is a crude antigen preparation that can be used to detect immediate- or delayed-type hypersensitivity to dermatophytic antigens. Many patients who develop chronic, noninflammatory dermatophyte infections have poor cell-mediated immune responses to dermatophyte antigen. These patients often are atopic and have immediate-type hypersensitivity and elevated IgE antibody levels. In the normal host, immunity to dermatophytosis varies in duration and degree depending on the host, the site, and the species of fungus causing the infection.
Dermatophyte infections were mistakenly described as ringworm or tinea because of the raised circular lesions, and tradition has maintained this terminology. The clinical forms are based on the site of involvement. A single species is able to cause more than one type of clinical infection. Conversely, a single clinical form, such as tinea corporis, may be caused by more than one dermatophyte species. Table 45-3 lists the more prevalent etiologies of common clinical forms. Very rarely, immunocompromised patients may develop systemic infection by a dermatophyte.
| Skin Disease | Location of Lesions | Clinical Features | Fungi Most Frequently Responsible |
|---|---|---|---|
| Tinea corporis (ringworm) | Nonhairy, smooth skin | Circular patches with advancing red, vesiculated border, and central scaling. Pruritic | Trichophyton rubrum, Epidermophyton floccosum |
| Tinea pedis (athlete’s foot) | Interdigital spaces on feet of persons wearing shoes | Acute: itching, red vesicular. Chronic: itching, scaling, fissures | T rubrum, Trichophyton mentagrophytes, E floccosum |
| Tinea cruris (jock itch) | Groin | Erythematous scaling lesion in intertriginous area. Pruritic | T rubrum, T mentagrophytes, E floccosum |
| Tinea capitis | Scalp hair. Endothrix: fungus inside hair shaft. Ectothrix: fungus on surface of hair | Circular bald patches with short hair stubs or broken hair within hair follicles. Kerion rare. Microsporum-infected hairs fluoresce | T mentagrophytes, Microsporum canis, Trichophyton tonsurans |
| Tinea barbae | Beard hair | Edematous, erythematous lesion | T mentagrophytes, T rubrum, Trichophyton verrucosum |
| Tinea unguium (onychomycosis) | Nail | Nails thickened or crumbling distally; discolored; lusterless. Usually associated with tinea pedis | T rubrum, T mentagrophytes, E floccosum |
| Dermatophytid (id reaction) | Usually sides and flexor aspects of fingers. Palm. Any site on body | Pruritic vesicular to bullous lesions. Most commonly associated with tinea pedis | No fungi present in lesion. May become secondarily infected with bacteria |
Tinea pedis is the most prevalent of all dermatophytoses. It usually occurs as a chronic infection of the toe webs. Other varieties are the vesicular, ulcerative, and moccasin types, with hyperkeratosis of the sole. Initially, there is itching between the toes and the development of small vesicles that rupture and discharge a thin fluid. The skin of the toe webs becomes macerated and peels, whereupon cracks appear that are prone to develop secondary bacterial infection. When the fungal infection becomes chronic, peeling and cracking of the skin are the principal manifestations, accompanied by pain and pruritus.
Nail infection may follow prolonged tinea pedis. With hyphal invasion, the nails become yellow, brittle, thickened, and crumbly. One or more nails of the feet or hands may be involved.
Dermatophytosis of the glabrous skin commonly gives rise to the annular lesions of ringworm, with a clearing, scaly center surrounded by a red advancing border that may be dry or vesicular. The dermatophyte grows only within dead, keratinized tissue, but fungal metabolites, enzymes, and antigens diffuse through the viable layers of the epidermis to cause erythema, vesicle formation, and pruritus. Infections with geophilic and zoophilic dermatophytes produce more irritants and are more inflammatory than anthropophilic species. As hyphae age, they often form chains of arthroconidia. The lesions expand centrifugally and active hyphal growth is at the periphery, which is the most likely region from which to obtain material for diagnosis. Penetration into the newly forming stratum corneum of the thicker plantar and palmar surfaces accounts for the persistent infections at those sites.
When the infection occurs in the groin area, it is called tinea cruris, or jock itch. Most such infections involve males and present as dry, itchy lesions that often start on the scrotum and spread to the groin. Tinea manus refers to ringworm of the hands or fingers. Dry scaly lesions may involve one or both hands, single fingers, or two or more fingers.
Tinea capitis is dermatophytosis or ringworm of the scalp and hair. The infection begins with hyphal invasion of the skin of the scalp, with subsequent spread down the keratinized wall of the hair follicle. Infection of the hair takes place just above the hair root. The hyphae grow downward on the nonliving portion of the hair and at the same rate as the hair grows upward. The infection produces dull gray, circular patches of alopecia, scaling, and itching. As the hair grows out of the follicle, the hyphae of Microsporum species produce a chain of spores that form a sheath around the hair shaft (ectothrix). These spores impart a greenish to silvery fluorescence when the hairs are examined under Wood’s light (365 nm). In contrast, T tonsurans, the chief cause of “black dot” tinea capitis, produces spores within the hair shaft (endothrix). These hairs do not fluoresce; they are weakened and typically break easily at the follicular opening. In prepubescent children, epidemic tinea capitis is usually self-limiting.
Zoophilic species may induce a severe combined inflammatory and hypersensitivity reaction called a kerion. Another manifestation of tinea capitis is favus, an acute inflammatory infection of the hair follicle caused by Trichophyton schoenleinii, which leads to the formation of scutula (crusts) around the follicle. In favic hairs, the hyphae do not form spores but can be found within the hair shaft. Tinea barbae involves the bearded region. Especially when a zoophilic dermatophyte is involved, a highly inflammatory reaction may be elicited that closely resembles pyogenic infection.
In the course of dermatophytosis, the patient may become hypersensitive to constituents or products of the fungus and develop allergic manifestations—called dermatophytids (usually vesicles)—elsewhere on the body, most often on the hands. The trichophytin skin test is markedly positive in such persons.
Specimens consist of scrapings from both the skin and the nails plus hairs plucked from involved areas. In KOH preps of skin or nails, regardless of the infecting species, branching hyphae or chains of arthroconidia (arthrospores) are seen (Figure 45-11). In hairs, most Microsporum species form dense sheaths of spores around the hair (ectothrix). The ectothrix spores of Microsporum-infected hairs fluoresce under Wood’s light in a darkened room. T tonsurans and Trichophyton violaceum are noted for producing arthroconidia inside the hair shaft (endothrix).
FIGURE 45-11
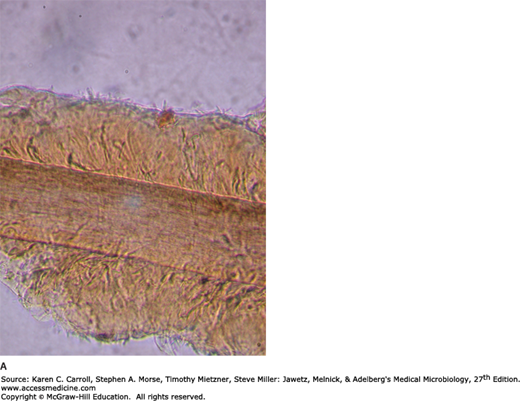
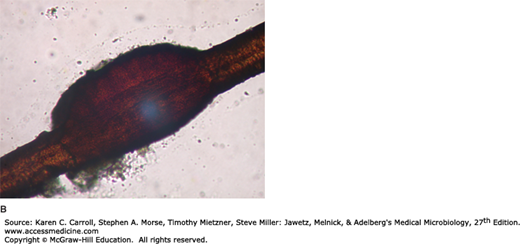
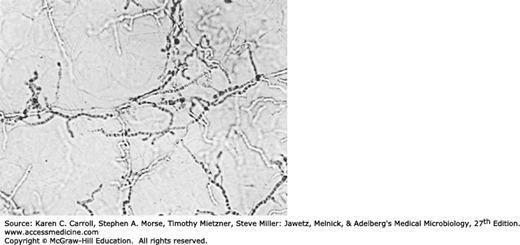
Dermatophytosis. Unstained microscopic KOH preparation of scrapings from a ringworm lesion. The epidermal cells are lysed by KOH to reveal hyaline branching septate hyphae. 100×. (Reproduced with permission from Ryan KJ, Ray CG [editors]: Sherris Medical Microbiology, 5th ed. McGraw-Hill, 2010, Figure 41-6, p. 700. © The McGraw-Hill Companies, Inc.)
The identification of dermatophytic species requires cultures. Specimens are inoculated onto IMA or SDA slants containing cycloheximide and chloramphenicol to suppress mold and bacterial growth, incubated for 1–3 weeks at room temperature, and further examined in slide cultures if necessary. Species are identified on the basis of colonial morphology (growth rate, surface texture, and any pigmentation), microscopic morphology (macroconidia, microconidia), and, in some cases, nutritional requirements.
Therapy consists of thorough removal of infected and dead epithelial structures and application of a topical antifungal drug. To prevent reinfection the area should be kept dry, and sources of infection, such as an infected pet or shared bathing facilities, should be avoided.
Scalp (tinea capitis) infections are treated for several weeks with oral administration of griseofulvin or terbinafine. Frequent shampoos and miconazole cream or other topical antifungal agents may be effective if used for weeks. Alternatively, ketoconazole and itraconazole are quite effective.
For tinea corporis, tinea pedis, and related infections, the most effective drugs are itraconazole and terbinafine. However, a number of topical preparations may be used, such as miconazole nitrate, tolnaftate, and clotrimazole. If applied for at least 2–4 weeks, the cure rates are usually 70–100%. Treatment should be continued for 1–2 weeks after clearing of the lesions. For troublesome cases, a short course of oral griseofulvin can be administered.
Nail infections (tinea unguium) are the most difficult to treat, often requiring months of oral itraconazole or terbinafine as well as surgical removal of the nail. Relapses are common. A new topical imidazole, luliconazole, has been formulated to penetrate the nail plate and demonstrated potent effectiveness against dermatophytes and onychomycosis.
KEY CONCEPTS: SUPERFICIAL AND CUTANEOUS MYCOSES
Superficial and cutaneous mycoses are among the most common of all communicable diseases.
Most superficial and cutaneous fungal infections are caused by species of Malassezia, dermatophytes, or Candida (discussed later).
The growth of dermatophytes is inhibited by serum and body temperature, and these fungi rarely become invasive.
Geophilic and zoophilic dermatophytes usually cause acute, inflammatory lesions that respond to topical treatment within weeks and rarely recur.
Anthropophilic dermatophytes usually cause relatively mild, chronic lesions that may require months or years of treatment and frequently recur.
SUBCUTANEOUS MYCOSES
The fungi that cause subcutaneous mycoses normally reside in soil or on vegetation. They enter the skin or subcutaneous tissue by traumatic inoculation with contaminated material. For example, a superficial cut or abrasion may introduce an environmental mold with the ability to infect the exposed dermis. In general, the lesions become granulomatous and expand slowly from the area of implantation. Extension via the lymphatics draining the lesion is slow except in sporotrichosis. These mycoses are usually confined to the subcutaneous tissues, but in rare cases they become systemic and produce life-threatening disease.
SPOROTRICHOSIS
Sporothrix schenckii is a thermally dimorphic fungus that lives on vegetation. It is associated with a variety of plants—grasses, trees, sphagnum moss, rose bushes, and other horticultural plants. At ambient temperatures, it grows as a mold, producing branching, septate hyphae and conidia, and in tissue or in vitro at 35–37°C as a small budding yeast. Following traumatic introduction into the skin, S schenckii causes sporotrichosis, a chronic granulomatous infection. The initial episode is typically followed by secondary spread with involvement of the draining lymphatics and lymph nodes.
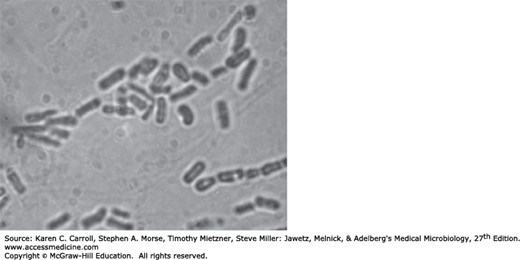
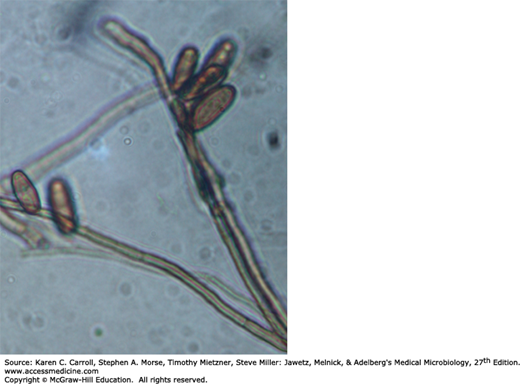
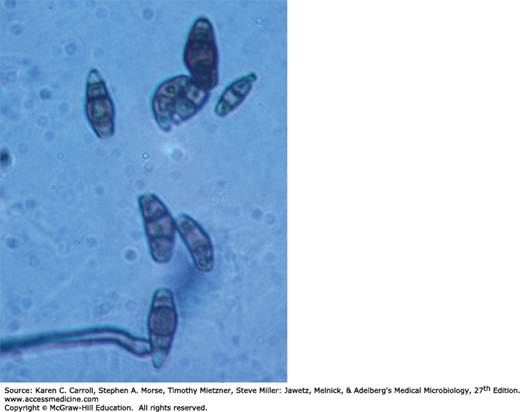
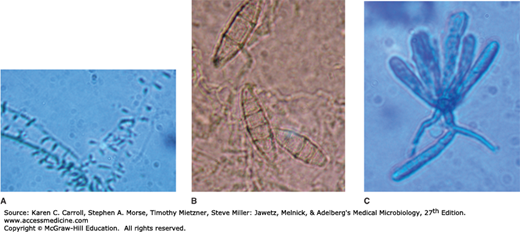
S schenckii grows well on routine agar media, and at room temperature the young colonies are blackish and shiny, becoming wrinkled and fuzzy with age. Strains vary in pigmentation from shades of black and gray to whitish. The organism produces branching, septate hyphae and distinctive small (3–5 μm) conidia, delicately clustered at the ends of tapering conidiophores. Isolates may also form larger conidia directly from the hyphae. S schenckii is thermally dimorphic, and at 35°C on a rich medium it converts to growth as small, often multiply budding yeast cells that are variable in shape but often fusiform (about 1–3 × 3–10 μm), as shown in Figure 45-12.
Heat-killed saline suspensions of cultures or carbohydrate fractions (sporotrichin) will elicit positive delayed skin tests in infected humans or animals. A variety of serologic tests have been developed, and most patients, as well as some normal individuals, have specific or cross-reactive antibodies.
The conidia or hyphal fragments of S schenckii are introduced into the skin by trauma. Patients frequently recall a history of trauma associated with outdoor activities and plants. The initial lesion is usually located on the extremities but can be found anywhere (children often present with facial lesions). About 75% of cases are lymphocutaneous; that is, the initial lesion develops as a granulomatous nodule that may progress to form a necrotic or ulcerative lesion. Meanwhile, the draining lymphatics become thickened and cord-like. Multiple subcutaneous nodules and abscesses occur along the lymphatics.
Fixed sporotrichosis is a single nonlymphangitic nodule that is limited and less progressive. The fixed lesion is more common in endemic areas such as Mexico, where there is a high level of exposure and immunity in the population. Immunity limits the local spread of the infection.
There is usually little systemic illness associated with these lesions, but dissemination may occur, especially in debilitated patients. Rarely, primary pulmonary sporotrichosis results from inhalation of the conidia. This manifestation mimics chronic cavitary tuberculosis and tends to occur in patients with impaired cell-mediated immunity.
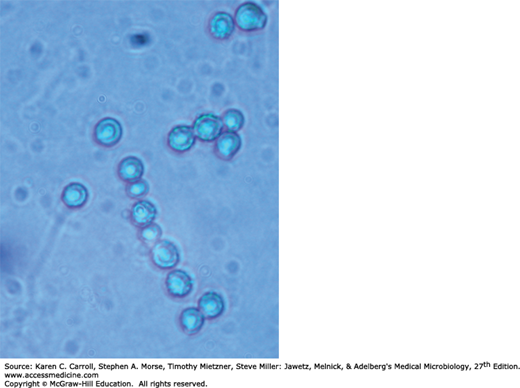
Specimens include biopsy material or exudate from granulomatous or ulcerative lesions. Although specimens can be examined directly with KOH or calcofluor white stain, the yeasts are rarely found. Even though they are sparse in tissue, the sensitivity of histopathologic sections is enhanced with routine fungal cell wall stains, such as Gomori methenamine silver, which stains the cell walls black, or the periodic acid-Schiff stain, which imparts a red color to the cell walls. Alternatively, they can be identified by fluorescent antibody staining. The yeasts are 3–5 μm in diameter and spherical to elongated.
Another structure termed an asteroid body is often seen in tissue, particularly in endemic areas such as Mexico, South Africa, and Japan. In hematoxylin and eosin-stained tissue, the asteroid body consists of a central basophilic yeast cell surrounded by radiating extensions of eosinophilic material, which are depositions of antigen–antibody complexes and complement.
The most reliable method of diagnosis is culture. Specimens are streaked on IMA or SDA containing antibacterial antibiotics and incubated at 25–30°C. The identification is confirmed by growth at 35°C and conversion to the yeast form.
High titers of agglutinating antibodies to yeast cell suspensions or antigen-coated latex particles are often detected in sera of infected patients. However, these tests are generally not useful because elevated titers do not develop early in the course of disease and uninfected or previously exposed patients may give false-positive results.
In some cases, the infection is self-limited. Although the oral administration of saturated solution of potassium iodide in milk is quite effective, it is difficult for many patients to tolerate. The treatment of choice is oral itraconazole or another azole. For systemic disease, amphotericin B is given.
S schenckii occurs worldwide in close association with plants. For example, cases have been linked to contact with sphagnum moss, rose thorns, decaying wood, pine straw, prairie grass, and other vegetation. About 75% of cases occur in males, either because of increased exposure or because of an X-linked difference in susceptibility. The incidence is higher among agricultural workers, and sporotrichosis is considered an occupational risk for forest rangers, horticulturists, and workers in similar occupations. Prevention includes measures to minimize accidental inoculation and the use of fungicides, where appropriate, to treat wood. Animals are also susceptible to sporotrichosis.
CHROMOBLASTOMYCOSIS
Chromoblastomycosis (chromomycosis) is a subcutaneous mycotic infection that is usually caused by traumatic inoculation of any of the recognized fungal agents, which reside in soil and vegetation. All are dematiaceous fungi, having melanized cell walls: Phialophora verrucosa, Fonsecaea pedrosoi, Fonsecaea compacta, Rhinocladiella aquaspersa, and Cladophialophora carrionii. The infection is chronic and characterized by the slow development of progressive granulomatous lesions that in time induce hyperplasia of the epidermal tissue.
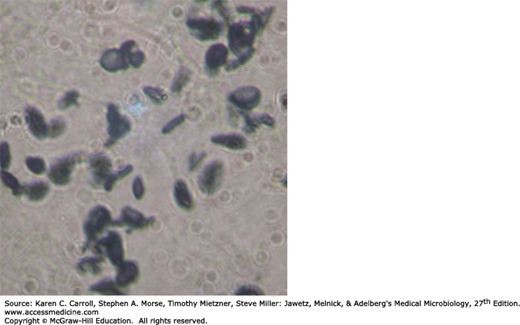
The dematiaceous fungi are similar in their pigmentation, antigenic structure, morphology, and physiologic properties. The colonies are compact, deep brown to black, and develop a velvety, often wrinkled surface. The agents of chromoblastomycosis are identified by their modes of conidiation. In tissue they appear the same, producing spherical brown cells (4–12 μm in diameter) termed muriform or sclerotic bodies that divide by transverse septation. Septation in different planes with delayed separation may give rise to a cluster of four to eight cells (Figure 45-13). Cells within superficial crusts or exudates may germinate into septate, branching hyphae.
Cultures of these dematiaceous molds can be distinguished as follows:
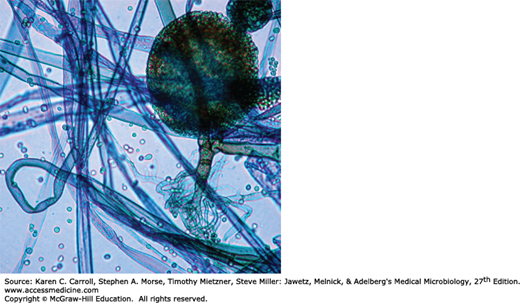
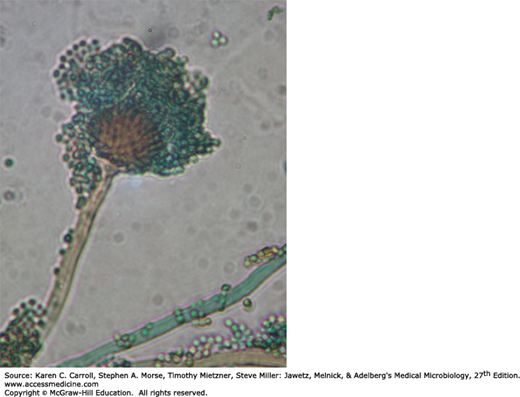
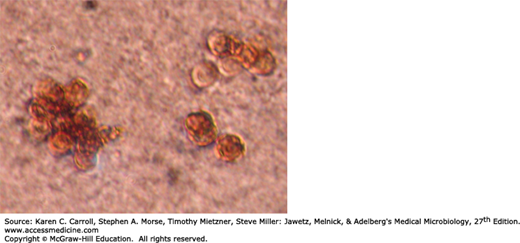
The conidia are produced from flask-shaped phialides with cup-shaped collarettes. Mature, spherical to oval conidia are extruded from the phialide and usually accumulate around it (Figure 45-14A).