Absence of family history
Aortic root dilatationa or aortic root dissection AND ectopia lentis
Aortic root dilatationa or aortic root dissection AND FBN1 mutation
Aortic root dilatationa or aortic root dissection AND systemic score ≥ 7 points (see Table 4.2)
Ectopia lentis AND FBN1 mutation that has been identified in an individual with aortic involvement
Presence of family history of Marfan syndrome
Aortic root dilatationb or aortic root dissection
Ectopia lentis
Systemic score ≥ 7 points
Table 4.2
Systemic features in Marfan syndrome
System | Manifestation | Points for systemic score |
|---|---|---|
Skeletal | Pectus carinatum | 2 points |
Pectus excavatum | Or chest asymmetry: 1 point | |
Scoliosis or spondylolisthesis | 1 point | |
Reduced upper to lower segment | When both are present without severe scoliosis. 1 point | |
Increased arm-span to height ratio (dolicostenomelia) | ||
Aracnodactilia | Wrist and thumb signs: both signs = 3 points; one sign = 1 point | |
Hindfoot deformity | 2 points | |
Pes planus | 1 point | |
Protrusio acetabulae | 2 points | |
Reduced extension of the elbows (<170°) | 1 point | |
Facial appearance: dolicocephaly, malar hypoplasia, enophtalmos, retrognathia, down-slanting palpebral fissures | In the presence of 3 of the 5 = 1 point | |
Highly arched palate with dental crowding | Not considered | |
Ocular | Ectopia lentis | Major criteria |
Myopia | > 3 diopters = 1 point | |
Retinal detachment | Not considered | |
Glaucoma | Not considered | |
Cardiovascular | Aortic dilatation with or without aortic regurgitation | At the level of aortic root is a major criteria (see Table 4.1) |
Aortic dissection | Ascending aorta dissection is a major criteria (see Table 4.1) | |
Mitral prolapse with or without mitral regurgitation | 1 point | |
Pulmonary artery dilatation | Not considered | |
Mitral annulus calcification in individuals younger than 40 years | Not considered | |
Pulmonary | Spontaneous pneumothorax | 2 points |
Apical blebs | Not considered | |
Integumentary | Stretch marks | 1 point |
Recurrent or incisional herniae | Not considered | |
Dura | Lumbosacral dural ectasia | By CT or MR: 2 points |
Limitations of genetic testing include the following: (1) the mutation in the fibrillin-1 gene can cause conditions other than Marfan-like disorders; (2) none of the current methods used to find mutations in the fibrillin-1 gene identify all mutations that cause MFS; and (3) family members with the same mutation causing MFS may present a wide range of clinical manifestations.
Complications
The main complication in patients with MFS is progressive aortic root enlargement, initially occurring at the sinuses of Valsalva. Ascending aortic aneurysm can precipitate acute type A aortic dissection or aortic rupture, and these complications were the primary cause of death before the advent of successful preventive therapies. Aortic aneurysm may develop early in children with MFS and the incidence rises during childhood and adolescence [3, 4]. Although early diagnosis has increased the median life span from around 40 to approximately 70 years, patients with MFS continue to suffer important morbidity [5]. Up to 90 % of Marfan patients will have cardiovascular events during their lifetime, including surgical repair of aortic root, aortic dissection or mitral valve surgery [6].
The current management of aortic involvement in MFS includes regular imaging follow-up to detect and quantify aortic dilation progression, and prophylactic aortic repair when aortic dilatation reaches a sufficient size sufficient to threaten dissection or cause aortic regurgitation. Prior to the era of open-heart surgery, the majority of patients with MFS died prematurely of aortic rupture, with an average life expectancy of 45 years [7]. The success of current medical and surgical treatment of aortic disease in MFS has substantially improved the average life expectancy, prolonging it up to 70 years [5, 8]. Thus, the major target for improving survival in patients with MFS is to prevent or delay aortic dissection.
Imaging Predictors of Complications
Several indices are associated with increased risk of a life-threatening aortic event. First among these is the absolute size of the proximal aorta [9, 10]. Aortic size ≥5.0 cm is strongly predictive of a high risk of aortic dissection and rupture [3], and surgical intervention at that stage is key. The “normal” diameter of the aorta is directly proportional to body size throughout normal growth and into adulthood. Given their above average stature and therefore greater body surface area, growing individuals with MFS should have their aortic measurements indexed to body surface area [10]. This can be expressed as an aortic size ratio based on sex- and body size–related norms or expressed in relation to the normal aortic size distribution in the population as a Z score. When considered in these terms, patients with MFS with proximal aortic ratios ≥1.3 or Z scores ≥3 are at particular risk. However, Marfan syndrome has interesting nuances. For example, adiposity is often reduced in young patients; therefore, the body surface area calculated from standard formulae will underestimate the expected diameters of the proximal aorta and result in a higher Z score. Moreover, adults tend to accumulate central adiposity in adulthood, which will increase the calculated body surface area and reduce the apparent degree of aortic dilatation. Adults who gain weight after skeletal maturity will appear to have an improved aortic Z score. In such instances, focus on the absolute diameter and its changes is appropriate. In addition, the existing “aortic growth curves” are divided into children and young adults; interestingly, the curves do not overlap accurately. This poses problems for the clinician managing patients passing from adolescence to adulthood. Additionally, a common question is whether tall adults should have larger aortic diameters, even beyond those considered to be normal. Svensson et al. [11, 12] proposed an index (area of aortic root/ height >10 cm2/m) to indicate surgery in patients with MFS. In addition to absolute aortic dimensions, the rate of change in size of the proximal aortic root over time is important. Even at relatively normal absolute aortic dimensions, a rapid increase in aortic size (>0.5 cm/year) portends an increased risk of dissection. However, to assume annual enlargement requires strict imaging quality control and re-measurement of aorta size at the same level and side by side. Additionally, a family history of early aortic complications is strongly predictive of decreased event-free survival [13]. Finally, diminished aortic compliance measured echocardiographically or by other means has been related to progressive aortic dilatation in MFS patients [14, 15], although it is rarely measured on a routine clinical basis. Also of importance is the fact that patients with MFS can die from other cardiovascular complications, particularly severe mitral regurgitation (especially in children with a severe phenotype) and dysrhythmia [16].
Pathophysiology of Aortic Dilatation
The earliest recognition of the tissue abnormalities underlying aortic dilatation in MFS was medial layer degeneration, with fragmentation, disarray and loss of elastic lamina, and replacement by basophilic-staining proteoglycan. Electron microscopy in humans and in a mouse model of MFS demonstrated extracellular matrix disarray, with shrunken smooth muscle cell fibres, thickened basal membranes, abnormalities of collagen fibre structure and progressive fragmentation and loss of elastic lamellae [17]. The process is associated with signs of ongoing inflammation and matrix metalloproteinase activation [18, 19].
Fibrillin-1 is a major protein component of the microfibrils in the extracellular matrix and, as a result of its alteration, fragmentation and disarray of elastic fibres occur. However, not all manifestations of MFS (e.g. bone overgrowth) can be attributed to these structural abnormalities. In recent years, basic research has led to the notion that fibrillin-1 microfibrils also exert significant regulatory effect on cytokin-transforming growth factor-β (TGF-β) [20].
TGF-β molecules are cytokines synthesised and secreted by smooth muscle cells as inactive precursors in the form of a latent complex which is stored in the extracellular matrix [21, 22]. The fibrillins and latent TGF-β–binding proteins constitute a family of structurally-related proteins and participate in the sequestration of latent complexes of TGF-β and maintain them inactive. In the presence of deficient fibrillin-1, a lesser amount of TGF-β is inactivated and leads to an increase in TGF-β activity. Excessive TGF-β signalling – made evident by increased smad-2 phosphorylation – explains many of the manifestations found in Marfan syndrome: cystic lungs, mixomatous mitral valve leaflets and aortic dilatation [20, 23].
Management
Although survival in these patients has improved dramatically in recent decades, mainly due to improved surgical techniques, most deaths in MFS patients are still due to aortic complications [5]. Routine aortic imaging by echo and/or MRI and CT is the recommended follow-up for these patients (Fig. 4.1), and elective aortic root surgery is considered when aortic root size is ≥50 mm [24]. However, medical treatment is needed to prevent aortic complications. As in other aortic conditions, strict blood pressure (BP) control is recommended. However, in MFS, medical treatment is considered to be prophylactic, even in the absence of high blood pressure, with the aim of reducing haemodynamic stress. The main aim of this chapter is to depict evidences, advantages and limitations of the current knowledge of the pharmacological treatment of this disease. To this end, several drugs will be discussed: β-blockers, angiotensin receptor blockers (ARB), angiotensin-converting enzyme inhibitors (ACEI) and calcium antagonists. More recent approaches such as statins, doxycycline, will also be reported.
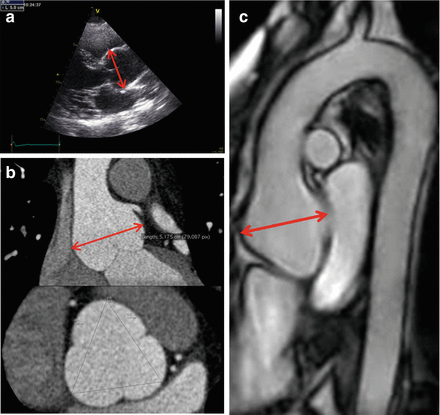

Figure 4.1
Imaging techniques for the study of the aorta in Marfan syndrome. (a) Transthoracic echocardiography; (b) computed tomography; (c) magnetic resonance imaging
Pharmacological Treatment in Marfan Syndrome
Mechanisms of Pharmacological Treatment
Medical treatment aims to reduce aortic haemodynamic stress: β-blockers, ARB, ACEI, calcium channel blocker (CCB), and/or to reduce TGF-β signalling: ARB. Recently, metalloproteinase inhibitors (MMPI) or anti-inflammatory drugs have been proposed.
Biomechanical and Haemodynamic Effects
Blood pressure and biomechanical properties of the aorta such as elasticity and compliance are determinant factors in aortic diameter enlargement in MFS [14, 15]. Different studies demonstrated that aortic stiffness is significantly greater in MFS patients compared with healthy volunteers, thereby suggesting more severe wall disease in MFS [25–29].
In clinical practice, arterial stiffness can be non-invasively estimated by three principal methods: (1) estimation of pulse wave velocity (PWV) by measurement of pulse transit time, (2) analysis of the arterial pressure wave contour (i.e. augmentation index, %), and (3) direct stiffness estimation using measurements of diameter or arterial luminal cross-sectional area change during the cardiac cycle and distending pressure measured at the site of diameter changes (i.e. distensibility and compliance). Carotid-to-femoral (‘aortic’) PWV is considered the gold standard [30] although PWV can also be measured at other levels.
β-blocker therapy reduces the exposure of weakened, histologically-abnormal aortic tissue to haemodynamic stressors by both inotropic and chronotropic negative effects, and thereby slows aortic dilatation progression. The use of β-adrenergic blockade to reduce haemodynamic stress in the proximal aorta in Marfan syndrome was first suggested in 1971, on the basis of findings in malignant hypertension that a reduction in the rate of increase in aortic pressure over time (dP/dt) was more effective at lowering the risk of aortic dissection than could be explained by a reduction of blood pressure alone [31]. Subsequent small studies of β-blockade effects in animal models with aortic disease and in uncontrolled studies of MFS had varying results [32]. β-blockers have proved to have little effect on central aortic pulse pressure in hypertensive patients [33], which is one of the main determinants of ascending aortic dilatation [34]. In 1989, Yin et al. [35] gave intravenous propranolol to Marfan subjects with dilated aortas during diagnostic cardiac catheterisation and found that it increased the magnitude of aortic wave reflection, reduced arterial compliance and did not reduce the maximum acceleration of blood into the ascending aorta. Other authors reported that β-blockade increases peripheral vascular resistance, which in turn may increase central aortic pressure and wall stress [36]. More recent studies also assessed the effect of β-blockers on aortic biomechanical properties: Groenink et al. [37] studied aortic properties by MRI and found a positive response of aortic distensibility and pulse wave velocity to the acute (2 weeks) treatment with metoprolol or atenolol; Rios et al. [36] found a heterogeneous response of aortic stiffness assessed by echocardiography to long-term treatment with atenolol. They defined a subgroup of patients in whom aortic distensibility improved after chronic β-blockade, with a more pronounced effect in Marfan patients with aortic root diameters below 40 mm. Furthermore, one study demonstrated that treatment with atenolol may not have an effect on the biomechanical properties of the aorta in paediatric patients with Marfan syndrome [38].
Recently, Nebivolol, a beta-1 receptor blocker with nitric oxide potentiating vasodilatory effects, has been proposed as a more appropriate choice than atenolol. In patients with hypertension, it reduces central pulse pressure and augmentation index more than atenolol, and it reduces central arterial pressure and left-ventricular hypertrophy more than metoprolol [39, 40].
Although one study assessed the role of aortic stiffness in predicting progressive aortic dilatation [14], the real clinical impact of the potential effect of β-blockade on aortic stiffness and aortic complications remains unclear.
Calcium-channel blockers reduce central aortic pressure in adult hypertensive patients [41], however similar effects have not been described in patients with MFS.
Angiotensin-converting enzyme inhibitors (ACEI) reduce angiotensin II (Ang-II) formation and are also known to reduce arterial stiffness in patients with different pathological conditions. More importantly, this ability seems to be independent of their ability to reduce BP. ACEI reduce central systolic pressure and conduit arterial stiffness, compared to β-blockers, in adults with hypertension [33].
One interesting study by Williams et al. [42] compared the haemodynamic and vascular effects of perindopril with those of two different drugs: atenolol and verapamil. Fourteen patients diagnosed of MFS were randomised (double-blinded) to receive 4 weeks of atenolol (75 mg), perindopril (4 mg) or verapamil (240 mg) in a cross-over design. Patients underwent a 2-week wash-out period prior to starting the protocol and after each treatment being switched to a new drug. Throughout the study, aortic diameter was assessed by transthoracic echocardiography, and arterial stiffness was measured as augmentation index and PWV (carotid-to-radial and carotid-to-femoral). Within-drug comparisons demonstrated that perindopril (−10.3 mmHg, P = 0.002), verapamil (−9.2 mmHg, P = 0.003) and atenolol (−7.1 mmHg, P = 0.01) reduced central systolic pressure and brachial pressure; central changes were the least and peripheral changes the greatest with atenolol; however between-drug comparisons were not significant. A trend was observed for augmentation to be reduced by perindopril (−6.3 %, P = 0.05), verapamil (−5.5 %, P = 0.07) and atenolol (−3.2 %, P = 0.09). The study results prove there were no statistically-significant differences among the drugs regarding aortic stiffness parameters. Only atenolol reduced heart rate (by 16 %) and delayed expansion in the arch and abdominal aorta (by 8 % and 11 %) (P < 0.001, P < 0.01 and P < 0.05, respectively, for inter-drug comparisons). Unexpectedly, atenolol did reduce central arterial pressure, although to a lesser degree than that observed with ACEI and CCB. This might be explained by a reduction in cardiac output (which fell by a mean of 17 %, P = 0.24) related to the reduction in heart rate (by a mean of 16 %, P = 0.006) rather than any change in stroke volume (12 %, P = 0.22). Alternatively, a negative inotropic effect would be expected to reduce the amplitude of aortic wave reflections during systole. This study suggested that a combination of a β-blocker with an ARB or an ACEI may be the most effective: while an ARB or ACEI may lower central pressures by reducing or delaying peripheral reflections, a β-blocker may reduce reflections by an effect on the left ventricle. This combination strategy is also being tested in some ongoing trials [43].
Molecular Effects
In order to reduce pathological molecular FBN1 mutation-derived mechanisms such as excessive TGF-β activation and signalling, different classes of drugs including ACEI and ARB have been investigated.
The creation of a mouse model of Marfan syndrome has significantly helped to further understanding of this disease. Overexpression of TGF-β explains many of the manifestations found in Marfan syndrome: cystic lungs, mixomatous mitral-valve leaflets and aortic dilatation have been associated with an increase in TGF-β signalling [20, 23]. Moreover, the administration of TGF-β antagonists (polyclonal TGF-β-neutralising antibody or losartan) in mice prevented the occurrence of Marfan features [44].
Inactive TGF-β is secreted by smooth muscle cells as a large latent complex. This latent complex is sequestered by the extracellular matrix and kept inactive. Deficient fibrillin-1 leads not only to histological abnormalities in the extracellular matrix microfibrils and connective tissue weakness, but also to a decrease in TGF-β sequestration leading to excessive TGF-β activation.
TGF-β can signal either through a canonical pathway involving the signal transduction proteins, Smads [45], or through several non-canonical, Smad-independent pathways (MAP-kinase pathway). In the Smad-related pathway, elevated TGF-β levels induce Smad2 activation that regulates transcription and induce the production of MMP proteins, a family of zinc endopeptidases responsible for degradation of the extracellular matrix in aortic aneurysms. The action of this class of proteins on aortic wall weakness in Marfan syndrome exponentially improves the risk of aortic aneurysm and rupture.
Ang–II is a potent vasoconstrictor acting directly on vascular smooth muscle cells and on the sympathetic nervous system; it also stimulates secretion of the hormone aldosterone, causing volume expansion through sodium retention. At molecular level, Ang-II can promote cell migration, proliferation and hypertrophy. Most of these effects are determined by Ang-II binding to its receptors: AT receptor 1 (AT1R) and AT receptor 2 (AT2R). Although angiotensin II (AngII) mediates the progression of aortic aneurysm, the relative contribution of its type 1 (AT1R) and type 2 (AT2R) receptors remains unknown. Ang-II promotes cell proliferation and fibrosis and suppresses apoptosis when binding to its AT1R, whereas binding to its AT2R has opposite effects, including antiproliferative and anti-inflammatory effects that are beneficial in aortic wall homeostasis. The effects of AT1R stimulation are mediated, at least in part, by TGF-β. The selective AT1 receptor blocker (ARB) losartan blocks AT1R and interferes with processes that are detrimental to tissue in mice with MFS (and by extension, humans) while not affecting signalling through AT2 that produces beneficial effects. ACEI, on the other hand, reduce Ang-II levels and therefore signalling through both receptors. Although both drugs proved to attenuate canonical TGF-β signalling in the aorta, only losartan inhibited TGF-β-mediated activation of extracellular signal–regulated kinase by allowing continued signalling through AT2.
Angiotensin-converting enzyme inhibitors (ACEI) prevent the conversion of angiotensin-I to Ang-II, thus limiting signalling through both AT receptors. On balance, however, it seems possible that the benefit of AT1-receptor antagonism achieved with ACE inhibitors could outweigh the potential negative influence of AT2-receptor blockade. Thus, although the rationale for the use of ACEI in Marfan syndrome includes their significant effect on TGF-β levels and activity, they proved to be less effective than the ARB losartan in a mouse model of MFS [46].
Treatment of affected mice with losartan, prenatally and continuing until 10 months of age, resulted in the preservation of proximal aortic elastic fibre histology and overall aortic diameter comparable to that of wild-type mice [44]. In contrast, mice with the same mutation treated with propranolol had elastic lamella disruption and dilated aortic roots comparable to those of affected mice treated with placebo [44]. When losartan therapy was initiated at 2 months of age, comparable to adolescence in humans, the histological abnormalities and dilatation were reversed. Although propranolol therapy was associated with a reduction in aortic growth rate, this effect was significantly less than that seen with losartan [44]. The results of this mouse model of MFS suggest that treatment with angiotensin receptor blockers potentially targets both the underlying tissue disorder and reduces haemodynamic stressors.
Telmisartan has the strongest binding affinity to AT1R in comparison with other ARBs including losartan [47]. Concretely, the rank order of binding affinity to AT1R is telmisartan > olmesartan > candesartan > valsartan ≥ losartan. If losartan achieves its effect on MFS through AT1R blockade mediated via downstream TGF-β signalling inhibition, telmisartan would be expected to be the most effective ARB because of its strongest binding affinity to AT1R. Future studies should determine, however, whether telmisartan is more effective than losartan in Marfan syndrome patients [48].
Matrix Metalloproteinase Inhibitors (MMPI) and Anti-inflammatory Drugs
Multiple factors such as haploinsufficiency, FBN1 proteolysis, abnormal TGF-β signalling, increased MMP expression and changes in cell matrix interaction contribute to the complex pathogenesis of this disorder. Collagens, laminins and elastin have multiple motifs that are able to interact with cell-surface receptors on macrophages and other inflammatory cells. Evidence is accumulating in support of the notion that inflammation may also play an important role in the development of thoracic aortic aneurysm in MFS.
Statins
HMG-CoA reductase inhibitors (statins) are the most potent class of drugs used to inhibit cholesterol biosynthesis. In addition to being the mainstay of cholesterol-lowering therapy, some studies reported more beneficial cardiovascular effects unrelated to lipid reduction, the so-called pleiotropic effects [49]. Interestingly, statins exert anti-inflammatory and atherosclerotic plaque stabilisation effects by downregulating matrix metalloproteinase (MMPs) expression [50]. Upregulation of MMP enzymes, particularly MMP-2 and MMP-9, is involved in MFS aortic wall degeneration and aneurysm formation [51].
Experimental research on a MFS animal model compared the effect of one of the statin family molecules, pravastatin, to losartan (angiotensin-2 antagonist). In that study, two Marfan genetically-modified mouse groups received, respectively, pravastatin 0.5 g/L and losartan 0.6 g/L for 6 weeks. Results from the different treated groups were compared with a third group of Marfan-modified untreated mice and a control group without pathological mutations. Echocardiogram analysis showed a significantly beneficial effect of pravastatin in attenuating aortic root dilatation in a MFS model (p < 0.01) compared to a Marfan untreated group. This outcome was analogous in the losartan group (p < 0.01). Moreover, immunohistochemical analysis of the mural architecture of the aortic wall demonstrated that pravastatin significantly reduced the degree of elastic fibres lost in the medial layer (p = 0.01). However, the losartan effect on elastin preserve was greater than that of statins (p < 0.01). In addition, haematoxylin and eosin staining showed the presence of foci of damage (island of damage) in the aortic wall of all MFS groups. Even if the number of foci was lower in treated animals, with no statistical difference between the medical groups, this finding may suggest that aortic injury was triggered in all groups and then reduced by drugs. Statins have been shown to have a potential role in MFS therapy and, therefore, this class of drugs should be investigated as a combination therapy in MFS patients.
Doxycycline
Doxycycline, a tetracycline-class antibiotic, is a non-specific inhibitor of MMPs [52] and suppresses aneurysm formation in animal models and human abdominal aortic aneurysm [53, 54]. In Marfan syndrome, Chung et al. [55] demonstrated that long-term treatment with doxycycline, through the inhibition of MMP-2 and −9, was more effective than atenolol in preventing TAA in a mouse model of Marfan syndrome by preserving elastic fibre integrity, normalising vasomotor function and suppressing TGF-β upregulation.
Indomethacin
The complex pathogenesis of MFS involves changes in TGF-β signalling, increased MMP expression and fragmentation of the extracellular matrix. A number of studies demonstrated raised macrophage and T-cell counts in the ascending aorta of human or mouse models of MFS; however, the efficacy of anti-inflammatory therapy in mouse MFS models has not been assessed to date. In a recent study, FBN1-underexpressing mgR/mgR Marfan mice were treated with oral indomethacin [56]. Treatment was begun at the age of three weeks and continued for 8 weeks, after which the aortas of wild type as well as treated and untreated mgR/mgR mice were compared. Indomethacin treatment led to a statistically-significant reduction in aortic elastin degeneration and macrophage infiltration, as well as lessening of MMP-2, MMP-9 and MMP-12 upregulation. Additionally, indomethacin reduced both cyclooxygenase-2 (COX-2) expression and activity in the aorta of mgR/mgR mice. COX-2-mediated inflammatory infiltrate contributed to aortic aneurysm progression in mgR/mgR mice, providing evidence that COX-2 is a relevant therapeutic target in MFS associated aortic aneurysmal disease. Therefore, COX-2-mediated inflammatory infiltration plays an important role in the pathogenesis of aortic aneurysm disease in MFS. In another paper, the same team demonstrated that the non-steroidal anti-inflammatory drug indomethacin significantly improved elastin integrity and reduced the number of macrophages in the aortic adventitia of mgR/mgR mice, which coincided with decreased MMP-2, MMP-9 and MMP-12 expression. Based on these studies, the authors speculated that the macrophage infiltration observed in the aortic wall of mgR/mgR Marfan mice participates in a kind of vicious cycle, in which matrix fragments induce deleterious effects, including upregulation of MMP activity and macrophage infiltration, which in turn reinforces the pathological processes associated with matrix degradation and defects in TGF-β sequestration [57–59].
Medical Treatment Studies
Beta-Blockers
Beta-blockers are the standard medical treatment for the prevention of aortic dilatation in Marfan syndrome. Their positive benefit relies on their haemodynamic effects: reduction in the force of left ventricular ejection by negative inotropic and chronotropic effects leading to decreased aortic wall stress. Several studies reported that β-blockers delay aortic root dilatation (Table 4.3). However, those studies had major limitations: the majority were retrospective [5, 60–63], and others prospective but not randomised [64, 65]. The majority showed retardation of aortic root dilatation [62, 66–69], although two studies did not demonstrate this benefit [61, 70]. None of those studies convincingly demonstrated a benefit in overall morbidity and mortality. The strongest evidence comes from a prospective randomised open-label trial by Shores et al. [66] that included 70 patients with Marfan syndrome divided into a control group of 38 patients who received no treatment and a treatment group of 32 patients who received propranolol. Aortic follow-up was performed by echocardiography and aortic dilatation was evaluated with the slope of the regression line for aortic ratio evolution over time. In that study, propranolol slowed the rate of aortic dilatation compared to the control group. The authors defined aortic ratio as the ratio of the measured aortic diameter to the expected diameter and the slope of the regression line for the increase in aortic ratios over time. The slope for aortic ratio of the control group was 0.084 per year, whereas in the treatment group was only 0.023 per year (p < 0.001). Five patients in the treatment group, two of whom did not follow the propranolol regimen, and nine patients in the control group reached a composite clinical end-point, which was defined as heart failure, aortic dissection, cardiovascular surgery or death. That study supported the use of β-blockers, concretely propranolol, in patients with Marfan syndrome based on two findings: first, aortic dilatation was faster in patients in the control group than in the treatment group and second, more patients in the control group reached the composite clinical end-point than in the treatment group. The construction of a composite end-point was necessary since no single clinical end-point reached statistical significance on its own merit. Although the results were certainly promising, the authors concede that the study was neither placebo-controlled nor blind, with each patient and investigator aware of the patient’s group. Thus, although the results did show potential for β-blockers in Marfan patients, it is highly possible that the study’s results were subject to bias and a placebo effect. Furthermore, although heart failure, dissection and death are hard end-points, the decision for surgery is a softer call and might have influenced the results.
Table 4.3
Studies on β-blockers in Marfan syndrome
Author | Design | Treatment groups | Age (years) | Mean aortic root at baseline | Includes children | Includes operated patients | Follow-up | Aortic dilatation end-points | Results |
|---|---|---|---|---|---|---|---|---|---|
Roman (1993) | Prospective observational Designed to assess the impact of aortic dilatation (no dilatation, localized to aortic root or generalized) and the presence of aortic complications | G1: β-blocker, not specified, N = 79 G2: None, N = 34 | 28 ± l5 yrs Range = 6 months – 66 yrs | Yes (n = 29) | No | 49 ± 24 months | Similar number of complication (AD, AAS, ARP) between groups | ||
Shores (1994) | Randomised clinical trial Open-label | G1: Propranolol, N = 32 G2: None, N = 38 | G1: 15.4 yrs G2: 14.5 yrs | G1: 34.6 mm G2: 30.2 mm Significantly different | Excluded <12 yrs | No | G1 = 10.7 yrs G2 = 9.3 yrs | Aortic root by echo (M-mode) Slope of the regression line for aortic ratio over time | Lower aortic dilatation by echo (M-mode) over time in treatment group (p < 0.001) Composite end-point (D, AD, AAS, CHF): 5 (15.6 %) in G1 and 9 in G2 (23.7 %). No statistical comparison reported |
Salim (1994) | Retrospective | G1 (Centre A or B): propranolol, atenolol, N = 100 G2: None, N = 13 | G1: Centre A: 10.4 ± 3.4 yrs Centre B: 14.1 ± 3.4 yrs G2: 0.2 ± 4.6 yrs | G1: Centre A: 31.1 ± 7.0 mm Centre B 34.0 ± 5.4 mm G2: 31.3 ± 7.4 mm P = NS | Yes | No | G1: Centre A: 5.5 ± 2.7 yrs Centre B: 4.2 ± 2.1 yrs G2: 5.7 ± 1.8 yrs | Aortic root dilatation rate by echo (M-mode) in mm/year | Aortic ratios (mm/year): Centre A 1.1 ± 1.1; Centre B 0.7 ± 1.8; Control 2.1 ± 1.6 (p < 0.006 between centre A and control: p < 0.003 between centre B and control) |
Silverman (1995) | Retrospective This study was designed to describe Marfan life expectancy compared to a historical cohort | G1: Atenolol, Nadolol, Propranolol, Metoprolol, N = 191 G2: None: N = 226 | G1: 33 ± 14 yrs G2: 31 ± 17 yrs | Yes | Yes | 5.2 ± 3.6 yrs | None | Median cumulative probability of survival 2 years longer in G1 (p = 0.01) | |
Legget (1996) | Retrospective Designed to examine the clinical and echocardiographic predictors of outcome in a cohort of patients with Marfan’s syndrome | G1: β-blocker, not specified, for >12 months, N = 30 G2: None, N = 53 | 21 ± 13 yrs Range 1–54 yrs | Yes | No | 4 yrs | Aortic ratios, by bidimensional echo | No differences in aortic root growth No differences in actuarial freedom from all events (D, AAS, AD) | |
Rossi-Foulkes (1999) | Prospective Non-randomized Open-label | G1: β-blockers, N = 20 G2: CCB, N = 6 G3: None, N = 27 | 9.4 ± 5.3 yrs | G1 + G2: 33 ± 7 mm G3: 26 ± 7 mm P < 0.01 No differences in aortic diameters indexed by BSA | Yes, exclusively | No | 44 ± 24 months | Aortic root dimensions by bidimensional echo (absolute values and aortic ratios) | Medicated patients had slower aortic growth than the unmedicated patients (both absolute aortic growth rate 1.0 ± 0.8 vs. 1.7 ± 1.0, p < 0.05) |
Selamet (2007) | Retrospective | G1: 27 atenolol, 1 propranolol and 1 metoprolol, N = 29 G2: None, N = 34 | G1: 9.2 ± 4.0 yrs G2: 8.8 ± 4.8 yrs | G1: 29.3 ± 4.2 mm G2: 29.7 ± 7 mm P = 0.75 | Yes, exclusively <18 yrs | No | G1: 76.3 ± 31.0 months G2: 81.3 ± 53.9 months | Aortic root dimensions by bidimensional echo (absolute values and z-scores) | No differences in aortic root growth Similar number of patients in each group achieved a clinical end-point (AAS, AD, D) |
Ladouceur (2007) | Retrospective | G1: Atenolol (70 %), nadolol (17 %) and propranolol (6 %), N = 77 G2: None, N = 78 | G1: 6.1 ± 3.2 yrs G2 7.4 ± 5.2 yrs | G1: 28.4 ± 4.8 mm G2: 27.2 ± 5.7 mm P = NS | Yes, exclusively <12 yrs | No | 4.5 ± 3.7 yrs | Aortic root dimensions by bidimensional echo | Aortic root dilatation 1.05 mm/year in G1 compared to 1.15 mm/year in G2, p = 0.0001 A trend toward lower cardiac mortality, decreased need for preventive aortic surgery, and less dissection was observed |



