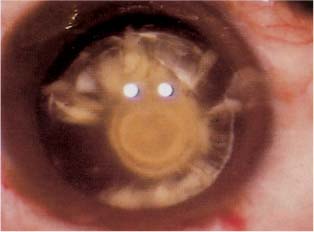
Chapter 15 The human crystalline lens is a unique structure. It is a cellular structure that loses its innervation and vascularity during fetal development. It derives its nutrition from the surrounding aqueous and vitreous humor. A disturbance in these fluids or in the substance of the lens may lead to metabolic abnormalities, culminating in the development of a cataract. The soft nucleus is usually cortical or subcapsular in nature. Cortical cataracts usually begin as water vacuoles and progress to transparent water clefts between cortical lamellae. The clefts become cloudy as they expand and imbibe water. They may begin peripherally and spread centrally, or as discrete water vacuoles, which proliferate throughout the substance of the nucleus. Histopathologically, water clefts are areas where the cortical lamellae are separated by swollen, degenerate lens fiber debris appearing as anuclear, pink, globular aggregates surrounded by paler pink, granular material. When the whole lens is involved it appears white and is classified as mature. At any point in the maturational process the lens may develop an osmotic gradient as a result of the increase in molecules due to breakdown of proteins. The resultant swelling is termed an intumescent cataract. When the brown endonucleus remains present, the cataract is classified as morgagnian. Subcapsular cataracts begin as a subtle sheen on the anterior or posterior capsule. They progress to white granular opacities and then enlarge to form a plaque of vacuoles and crystals. The plaque may thicken. Histopathologically, this type of cataract is associated with posterior migration of lens epithelial cells. The cells then become larger, more spindled, and fibroblast-like. These cells then surround the liquefying posterior cortex in a ring.1 The abnormal lens epithelial cells may then grow into the posterior capsule, causing a ring-like posterior capsular fibrotic plaque. The plaque is fused to the posterior capsule and cannot be removed surgically without tearing the capsule. When it is present, rather than attempt operative removal, the plaque should be polished intraoperatively as best as possible, and the procedure completed. Yttrium-aluminum-garnet (YAG) capsulotomy can then be performed as early as 2 weeks postoperatively, although a longer period of observation is advantageous to allow stabilization of the blood–aqueous barrier prior to laser. Posterior subcapsular cataracts are associated with diabetes mellitus, topical or systemic steroid use, trauma, inflammation, and irradiation.2,3 A unique type of posterior subcapsular cataract is the posterior polar cataract (Fig. 15–1). This cataract is often inherited as autosomal dominant.4,5 In this type of cataract a dense white opacity occurs adjacent to the central posterior capsule. The opacity may be stationary or progressive, with cortical extensions. Concentric thickened rings around the central opacity give the impression of a bull’s-eye.6 The posterior capsule beneath the opacity is reportedly thin and prone to rupture. Osher et al7 report that one-quarter of these cataracts are associated with capsular rupture. Dense white satellite opacities adjacent to the bull’s-eye may indicate a preexisting capsular dehiscence.8 Calcification is an almost sure sign of posterior capsular involvement and of the likelihood of capsular rupture during cataract surgery. FIGURE 15–1
MANAGEMENT OF THE
SOFT NUCLEUS
ANATOMY AND PATHOPHYSIOLOGY
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree