EPIDEMIOLOGY OF TRAUMA
As a “disease,” trauma is a major public health problem. In the United States, it is the leading cause of death among people aged 1-45. For persons under age 30, trauma is responsible for more deaths than all other diseases combined. Because trauma adversely affects a young population, it results in the loss of more working years than all other causes of death. Presence of alcohol is a significant contributor to trauma fatalities, and one-third of all traffic deaths are alcohol related. The financial costs of injury are astounding and exceed $400 billion annually. Regrettably, nearly 40% of all trauma deaths could be avoided by injury prevention measures (50% of passenger vehicle occupants killed were unrestrained), alcohol cessation, and by the establishment of regional trauma systems that would expedite the evaluation and treatment of seriously injured patients.
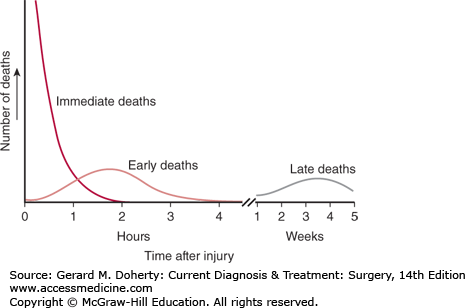
Trauma deaths have been described as having a trimodal distribution (Figure 13–1), with peaks that correspond to the types of intervention that would be most effective in reducing mortality. The first peak, the immediate deaths, represents patients who die of their injuries before reaching the hospital. The injuries accounting for these deaths include major brain or spinal cord trauma and those resulting in rapid exsanguination. Few of these patients would have any chance of survival even with access to immediate care because almost 60% of these deaths occur at the same time as the injury. Prevention remains the major strategy to reduce these deaths.
The second peak, the early deaths are those that occur within the first few hours after injury. Half are caused by internal hemorrhage, and the other half, by central nervous system injuries. Almost all of these injuries are potentially treatable. However, in most cases, salvage requires prompt and definitive care of the sort available at a trauma center, which is a specialized institution that can provide immediate resuscitation, identification of injuries, and access to a ready operating room 24 hours a day. Development of well-organized trauma systems with rapid transport and protocol-driven care can reduce the mortality in this time period by 30%.
The third peak, the late deaths, consists of patients who die days or weeks after injury. Ten percent to 20% of all trauma deaths occur during this period. Mortality for this period has traditionally been attributed to infection and multiple organ failure. However, development of trauma systems has changed the epidemiology of these deaths. During the first week, refractory intracranial hypertension following severe head injury is now responsible for a significant number of these deaths. Improvements in critical care management continue to be essential in reducing deaths during this phase. It is paramount that surgeons caring for trauma patients have genuine expertise in surgical critical care.
TRAUMA SYSTEMS
The terrorist attacks of September 11, 2001, highlighted the need for national and state trauma systems that can handle both routine events and mass casualty situations. The purpose of a trauma system is to provide timely, organized care in order to minimize preventable morbidity and mortality following injury. The system includes prehospital care designed to identify, triage, treat, and transport victims with serious injuries. Criteria for staging patients with major trauma consist of standardized scoring systems based on readily discernible anatomic and physiologic variables. These criteria are designed to identify not only the more severe and complex single injuries but also combinations of injuries that require tertiary care. Trauma centers that are part of a larger trauma system are already organized to respond to unexpected multiple casualty events. Regional trauma centers have established links with emergency medical service providers and participate in systemwide patient triage and quality improvement. Gaps remain, however, in areas of the country not served by trauma systems, and a wide range in the degree of disaster preparedness exists at all levels of trauma care.
The American College of Surgeons (ACS) defines four levels of institutional trauma care. Level I is the highest designation a trauma center can receive. It indicates that the hospital has committed itself to the care of trauma patients and offers the highest level of skill available in trauma care. A level I trauma center is directed by a board-certified surgeon specializing in trauma care and is staffed by a team of board-certified trauma care specialists available 24 hours a day—including emergency room physicians, trauma surgeons, neurosurgeons and neurologists, orthopedic surgeons, plastic surgeons, anesthesiologists, and radiologists. Level II trauma centers provide 24-hour care by in-hospital and on-call physicians. They can deliver the same quality of care as a level I center but without the same teaching and research obligations. The level III trauma center provides prompt assessment, resuscitation, and stabilization followed by surgical treatment or interhospital transfer as appropriate. Level III centers serve a valuable function in less populated areas where resuscitation and stabilization before transport may be lifesaving. Level IV centers are designed to provide advanced trauma life support (ATLS) prior to patient transfer in remote areas in which no higher level of care is available.
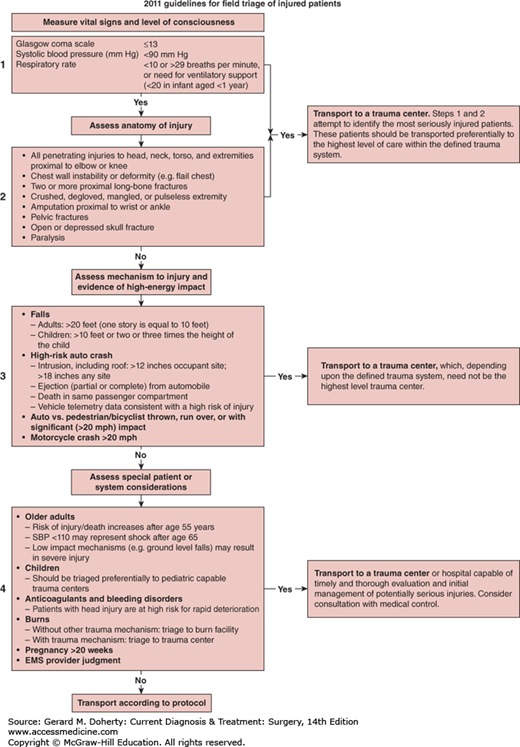
Determining if a victim of a vehicle crash requires care at a trauma center is a decision for emergency medical responders. The Centers for Disease Control (CDC) has sponsored research and policy making aimed at providing guidance to answer this question. It is established that care at a level 1 trauma center reduces the risk of death by 25% for severely injured patients, when compared to treatment at a hospital which does not have verified trauma center resources. The CDC has undertaken two initiatives based on research and expert panel findings: (1) guidelines for the field triage of injured patient and (2) advanced automatic collision notification and triage of the injured patient (AACN). The published guidelines for the field triage of injured patients provides a rapid easily understood algorithm which allows for determination of where to triage trauma patients based upon findings at the scene taking into account assessment of vital signs/level of consciousness, anatomic evidence of injury, and mechanism of injury with regard to the level of energy involved (Figure 13–2). Partnering with the automotive industry has allowed for development of sophisticated sensors and vehicle telematics that allow early notification of a crash, prediction of the likelihood of serious injury among the occupants, and assistance with the field triage decision making. In combination, the AACN system and the field triage algorithm are expected to decrease the time that it takes for a seriously injured patient to receive appropriate and definitive trauma care.
PREHOSPITAL CARE & IMMEDIATE MEASURES AT A CRASH SITE
At first glance, the trauma victim may not appear to be badly injured. Sometimes there may be little external evidence of injury; however, when the mechanism of trauma is sufficient to produce severe injury, the victim must be handled as if a severe injury has occurred. It is critical at the scene that the injured person be protected from further trauma; likewise, rescue personnel need to take precautions to avoid injury to themselves. First aid at the scene of an accident should be administered by trained personnel whenever possible.
Whether the patient is first seen on the battlefield, beside a road, in the emergency ward, or in the hospital, the basic principles of initial management are the same:
Is the victim breathing? If not, provide an airway and establish bag-mask ventilation.
Is there a pulse or heartbeat? If not, begin closed-chest compression.
Is there gross external bleeding? If so, elevate the part if possible and apply enough external pressure to stop the bleeding. A tourniquet when utilized by trained providers is acceptable in circumstances of extreme bleeding.
Is there any question of injury to the spine? If so, protect the neck and spine before moving the patient.
Splint obvious fractures.
As soon as these steps have been taken, the patient can be safely transported.
EVALUATION OF THE TRAUMA PATIENT
In most situations, a brief history is obtained from prehospital personnel via radio communication or when the patient arrives at the hospital. In the case of motor vehicle crashes, for example, it is important to determine the circumstances of the injury, including the speed of impact, the condition of the vehicle, the position of the patient at the scene, type of restraint systems present, evidence of blood loss, and the condition of other passengers. The time that the injury occurred and the treatment rendered while en route is recorded. Knowing the mechanism of injury often gives a clue to concealed trauma. Information regarding serious underlying medical problems should be sought from Medic Alert bracelets or wallet cards. If the patient is conscious and stable, the examiner should obtain a complete history and use this information to direct the examination in order to avoid unnecessary tests.
Trauma victims require a precise, rapid, systematic approach to initial evaluation in order to ensure their survival. The ATLS system developed by the ACS Committee on Trauma represents the best current approach to the severely injured patient. The sequence of evaluation includes primary survey, resuscitation, secondary survey, and definitive management. The primary survey attempts to identify and treat immediate life-threatening conditions. Resuscitation is performed, and the response to therapy is evaluated. The secondary survey includes a comprehensive physical examination designed to detect all injuries and establish a treatment priority for potentially life-threatening and/or limb-threatening ones. During the primary and secondary surveys, appropriate laboratory and imaging studies are performed to aid in the identification of injuries and prepare the patient for definitive care.
The ATLS manual and provider course published by the ACS Committee on Trauma is the accepted guideline for the primary survey. The primary survey is a rapid assessment to detect life-threatening injuries following the ABCDE: airway, breathing, circulation, disability, and exposure/environment.
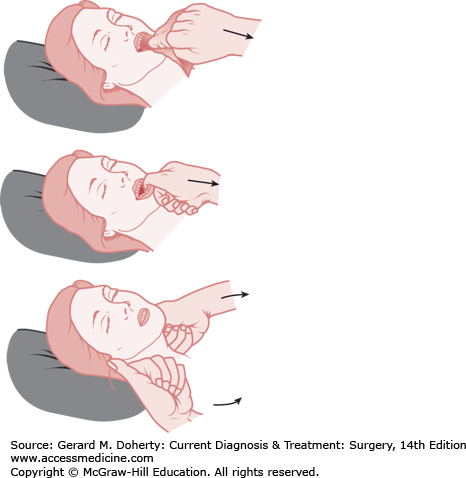
The establishment of an adequate airway has the highest priority in the primary survey. Oxygen by high-flow nasal cannula (10-12 L/min), 100% nonrebreather mask, or bag-mask ventilation with pulse oximetry should be started if not already in place. Maneuvers used in the trauma patient to establish an airway must consider a possible cervical spine injury. Any patient with multisystem trauma, especially those with an altered level of consciousness or blunt trauma above the clavicles, should be assumed to have a cervical spine injury. The rapid assessment for signs of airway obstruction should include inspection for foreign bodies and facial, jaw, or tracheal/laryngeal fractures that may result in acute loss of airway patency. Techniques that can be used to establish a patent airway while protecting the cervical spine include the chin lift or jaw thrust maneuvers (Figure 13–3).
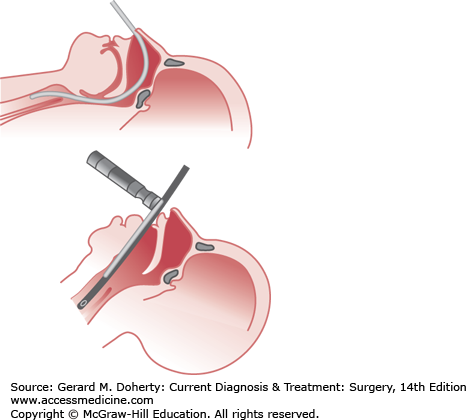
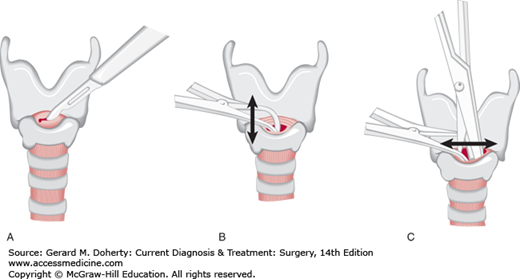
Patients who can communicate verbally without difficulty are unlikely to have an impaired airway. Repeated assessment of airway patency is always prudent. Those patients with severe head injury, altered level of consciousness, or Glasgow Coma Scale (GCS) score 8 or less usually require placement of a definitive airway. Orotracheal or nasotracheal intubation can be attempted with cervical spine precautions if a second person maintains axial immobilization of the head to prevent destabilization of the spine (Figure 13–4). If ventilatory failure occurs and an adequate airway cannot be obtained readily by orotracheal or nasotracheal intubation, surgical cricothyroidotomy should be performed as rapidly as possible (Figure 13–5).
Once the airway has been established, it is necessary to ensure that oxygenation and ventilation is adequate. Examine the patient to determine the degree of chest expansion, breath sounds, tachypnea, crepitus from rib fractures, subcutaneous emphysema, and the presence of penetrating or open wounds. Immediately life-threatening pulmonary injuries that must be detected and treated promptly include tension pneumothorax, open pneumothorax, flail chest, and massive hemothorax. Chest injury has the second highest case fatality rate in the trauma patient. The following are examples of life-threatening pulmonary injuries and their treatment:
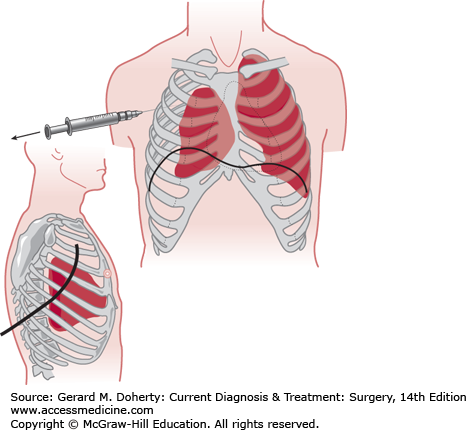
This condition occurs when air becomes trapped in the pleural space under pressure. The harmful effects result primarily from shift of the mediastinum, impairment of venous return, and potential occlusion of the airway. Tension pneumothorax is difficult to diagnose even when the patient reaches the hospital. The clinical findings consist of hypotension in the presence of distended neck veins, decreased or absent breath sounds on the affected side, hyperresonance to percussion, and tracheal shift away from the affected side. These signs may be difficult to detect in a hypovolemic patient with a cervical collar in place. Cyanosis may be a late manifestation. Emergency treatment consists of insertion of a large-bore needle or plastic intravenous cannula (angiocath) through the chest wall into the pleural space in the second intercostal space along the midclavicular line to relieve the pressure and convert the tension pneumothorax to a simple pneumothorax. The needle or cannula should be left in place until a thoracostomy tube is inserted for definitive management (Figure 13–6).
This condition results from an open wound of the chest wall with free communication between the pleural space and the atmosphere. The resulting impairment of the thoracic bellows, and its ability to expand the lung results in inadequate ventilation. With chest expansion during a breath, air moves in and out of the chest wall opening instead of through the trachea, producing hypoventilation that can be rapidly fatal. Emergency treatment consists of sealing the wound with an occlusive sterile dressing taped on three sides to act as a flutter-type valve or with any material if nothing sterile is available. Definitive treatment requires placement of a chest tube to reexpand the lung and surgical closure of the defect. Airway intubation with positive-pressure mechanical ventilation can be helpful in massive open pneumothorax.
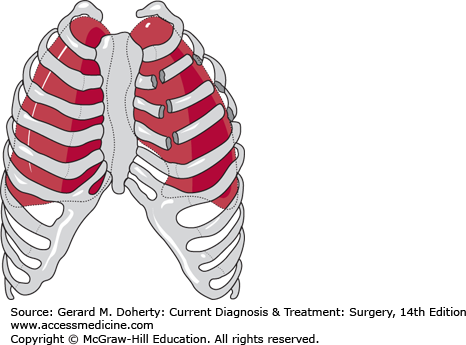
Multiple rib fractures resulting in a free-floating segment of chest wall may produce paradoxical motion that impairs lung expansion (Figure 13–7). In patients with flail chest, injury-associated pulmonary contusion is common and is often the major cause of respiratory failure. The injury is identified by careful inspection and palpation during physical examination. Patients with large flail segments will almost always require endotracheal intubation and mechanical ventilation both to stabilize the flail segment and to optimize gas exchange. Smaller flail segments may be well tolerated if supplemental oxygen and adequate analgesia are provided. The work of breathing is increased considerably, and many patients who initially appear to be compensating well may suddenly deteriorate a few hours later. Therefore, most patients with flail chest require monitoring in an intensive care unit (ICU).
Figure 13–6.
Relief of pneumothorax. Tension pneumothorax must be immediately decompressed by a needle introduced through the second anterior intercostal space. A chest tube is usually inserted in the midaxillary line at the level of the nipple and is directed posteriorly and superiorly toward the apex of the thorax. The tube is attached to a “three-bottle” suction device, and the rate of escape of air is indicated by the appearance of bubbles in the second of the three bottles. Cessation of bubbling suggests that the air leak has become sealed.
Free hemorrhage from accessible surface wounds is usually obvious and can be controlled in most cases by local pressure and elevation of the bleeding point. Firm and precise pressure on the major artery in the axilla, antecubital space, wrist, groin, popliteal space, or at the ankle may suffice for temporary control of arterial hemorrhage distal to these points. When all other measures have failed, a tourniquet may be necessary to control major hemorrhage from extensive wounds or major vessels in an extremity. Failure to manage a tourniquet properly may cause irreparable vascular or neurologic damage. For this reason, the tourniquet should be used only when necessary and must be kept exposed and loosened at least every 20 minutes for 1 or 2 minutes while the patient is in transit. Transport to definitive care where the tourniquet can be safely removed and treatment rendered should be a top priority. It is wise to write the time of tourniquet application in military time on the patient’s forehead with a skin-marking pen or on adhesive tape.
All patients with significant trauma should have large-caliber peripheral intravenous catheters inserted immediately for administration of crystalloid fluids as needed. If any degree of shock is present, at least two 14-16 gauge peripheral intravenous lines should be established usually in the antecubital fossa. If venous access cannot be obtained by percutaneous peripheral or central venous cannulation, an intraosseous line in the tibia or other uninjured site should be placed. A venous cutdown of the saphenous vein at the ankle using an angiocath or intravenous extension tubing with the tip cut off can be performed, but is a difficult procedure in the field. A blood sample for type and cross-match should be sent from the venous line, if not already drawn.
As soon as the first intravenous line is inserted, rapid crystalloid infusion should begin. Adult patients should be given 2 L of Ringer lactate or normal saline. For children, the initial administered crystalloid volume should be 20 mL/kg. Patients who experience a transient response should receive an additional infusion of 2 L of crystalloid. Patients in hemorrhagic shock for whom there is no improvement in blood pressure from initial crystalloid infusion or who transiently respond but fail a second crystalloid bolus should be switched to resuscitation with blood products. Beyond the administration of the first two units of packed red blood cells (PRBC), it is important to also administer fresh plasma or thawed fresh frozen plasma (FFP) to avoid coagulopathy in the massively transfused patient. Massive transfusion is defined as at least 10 units of PRBC. The exact ratio of FFP to PRBC is still under active investigation, but a target range of 1:1 or 2:3 is considered acceptable. Military data has shown that the early use of plasma can reduce mortality by up to 50% in the massively transfused trauma patient. Giving platelets as part of the massive transfusion protocol is also supported by military data demonstrating a 20% reduction in mortality for patients who received platelets as fresh whole blood or apheresis platelets in conjunction with PRBC. The exact platelet-to-PRBC ratio for optimal treatment of the hemorrhaging trauma patient is not known, but giving a unit of platelets for every five units of PRBC is a reasonable ratio. Type O, Rh-negative PRBC should be immediately available in the emergency department for any patient with impending cardiac arrest or massive hemorrhage. Some high-volume trauma centers now also stock fresh or “prethawed” AB plasma as well. Type-specific blood should be available within 15-20 minutes of patient arrival to the hospital.
Tranexamic acid is an antifibrinolytic agent which inhibits the breakdown of clotted blood. It does not promote new blood clot formation. Two large clinical trials, one in the military setting and another in civilian population, have demonstrated the efficacy of tranexamic acid when utilized as part of a blood product resuscitation strategy in patients with traumatic hemorrhage. The survival benefit is greatest in patients who received massive transfusion and those in whom early treatment was accomplished (≤ 1 hour after injury). Tranexamic acid started greater than 3 hours after injury increased the risk of death due to bleeding and is likely ineffective. Dosing is typically 1 g intravenously over 10 minutes, followed by a drip infusion of an additional 1 g over 8 hours.
Transfusion of blood products is not without risk. Despite rigorous screening programs, transmission of viral blood-borne diseases can occur. The current incidence of blood-borne pathogen transmission following red blood cell transfusion are hepatitis B, 1:350,000; hepatitis C 1:400,000; and HIV, 1:2 million. Transfusion of blood products is also associated with transfusion-related immunomodulation and transfusion-related acute lung injury. Both of these problems can increase morbidity and mortality. The storage age of blood transfused can also contribute to problems. Transfusion of a patient with older units of PRBC has been shown to cause generation of systemic proinflammatory mediators and increase the risk of wound infection.
As intravenous access is obtained, electrocardiogram leads for continuous cardiac monitoring should be placed. Noninvasive blood pressure measurements should be acquired with a time-cycled blood pressure cuff. Pulse oximetry is valuable in ensuring that adequate hemoglobin oxygen saturation is present in the injured patient. Temperature is a crucial vital sign, and it should be measured and recorded along with the first pulse and blood pressure in the emergency department.
A brief neurologic examination should be documented to assess patients’ degree of neurologic impairment. Many factors may contribute to altered levels of consciousness and should be considered in addition to central nervous system injury in all trauma patients. Other than the direct trauma, the most common contributing causes of altered mental status for trauma patients are alcohol intoxication, other central nervous system stimulants or depressants, diabetic ketoacidosis, cerebrovascular accident, and hypovolemic shock. Less common causes are epilepsy, eclampsia, electrolyte imbalances associated with metabolic and systemic diseases, anaphylaxis, heavy metal poisoning, electric shock, tumors, severe systemic infections, hypercalcemia, asphyxia, heat stroke, severe heart failure, and hysteria. These uncommon causes of coma or diminished mental status should be considered if routine testing such as blood alcohol and glucose level, urine toxicology, and head computerized tomography (CT) scanning are unrevealing as to the etiology of mental impairment. In such cases, further laboratory and diagnostic testing may be warranted.
The differential diagnosis depends upon a careful history and complete physical examination, with particular attention to the neurologic examination with documentation of the patient’s GCS score (Table 13–1), and an urgent head CT scan. The GCS score is useful in monitoring acute changes in neurologic function and is used for prognosticating outcomes after severe head injury. The motor component of the GCS score is the most accurate for predicting outcome and has a linear relationship with mortality. Lateralizing signs may also suggest evidence of an intracranial mass effect or carotid or vertebral artery injury, while loss of distal motor and/or sensory function may help localize potential spinal cord injuries.
All clothing should be removed (cut off with trauma shears, usually) from the seriously injured patient, with care taken to avoid unnecessary movement. The removal of helmets or other protective clothing may require additional personnel to stabilize the patient and prevent further injury. All skin surfaces should be examined to identify occult injuries that may not be readily apparent, such as posterior penetrating trauma or open fractures. After inspecting all surfaces, warm blankets or warming devices should be placed to avoid hypothermia in the seriously injured patient.
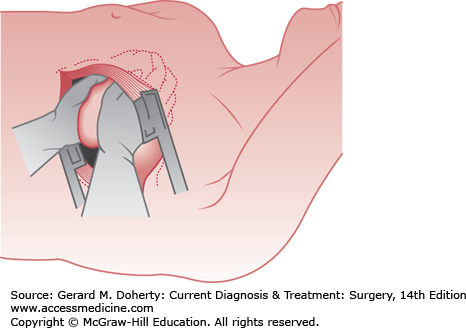
Certain injuries are so critical that operative treatment must be undertaken as soon as the diagnosis is made. In these cases, resuscitation is continued as the patient is being operated on. For cardiopulmonary arrest that occurs in the emergency room as a direct result of trauma, external cardiac compression is rarely successful in maintaining effective perfusion of vital organs. An emergency left anterolateral thoracotomy should be performed in the fourth or fifth intercostal space, and the pericardium should be opened anterior to the phrenic nerve (Figure 13–8). Open cardiac massage, cross-clamping of the descending thoracic aorta, repair of cardiac injuries, and internal defibrillation can be performed as appropriate. Wounds of the lung producing severe hemorrhage or systemic air embolus may require pulmonary hilar cross-clamping.
Emergency room thoracotomy is most useful for cardiac arrest due to penetrating thoracic trauma, particularly in patients with pericardial tamponade from stab wounds. This extreme procedure is ineffective for most patients with cardiac arrest due to blunt trauma and for all patients who have no detectable vital signs in the field (< 1% survival). If vital signs are present in the emergency room but arrest appears imminent, the patient should be transferred rapidly to the operating room if at all possible, since conditions in the operating room are optimal for surgical intervention.
Shock is defined as inadequate end-organ tissue perfusion. Some degree of shock accompanies most severe injuries and is manifested initially by pallor, cold sweat, weakness, lightheadedness, tachycardia, hypotension, thirst, air hunger, and, eventually, loss of consciousness. Patients with any of these signs should be presumed to be in shock and evaluated thoroughly. All patients determined to be in any degree of shock should be reexamined at regular intervals. The degree of shock has been categorized to guide resuscitation and help caregivers recognize the severity of symptoms (Table 13–2).
| Class I | Class II | Class III | Class IV | |
|---|---|---|---|---|
| Blood loss (mL) | Up to 750 | 750–1500 | 1500–2000 | > 2000 |
| Blood loss (% BV) | Up to 15% | 15–30% | 30–40% | > 40% |
| Pulse rate (beats/min) | < 100 | > 100 | > 120 | > 140 |
| Blood pressure | Normal | Minimal decrease | Decreased | Significantly decreased |
| Pulse pressure | Normal | Narrowed | Narrowed | Unobtainable or very narrow |
| Hourly urine output | ≥ 0.5 mL/kg | ≥ 0.5 mL/kg | < 0.5 cc/kg | Minimal |
| CNS/mental status | Slightly anxious | Mildly anxious | Anxious & confused | Confused or lethargic |
Hypovolemic shock is due to loss of whole blood or plasma. Blood pressure may be maintained initially by vasoconstriction. Tissue hypoxia increases when hypotension ensues, and shock may become irreversible if irreparable damage occurs to the vital organs. Massive or prolonged hemorrhage, severe crush injuries, major fractures, and extensive burns are the most common causes. The presence of any of these conditions is an indication for prompt intravenous fluid infusion.
In mild or class 1 shock (< 15% blood volume loss), compensatory mechanisms may preserve adequate perfusion, and no skin or physiologic changes may be apparent. In moderate or class 2 shock (15%-30% blood loss), the skin on the extremities becomes pale, cool, and moist as a result of vasoconstriction and release of epinephrine. Systolic blood pressure is often maintained at near-normal levels, but urine output will usually decrease. With severe or class 3 shock (30%-40% blood volume loss), these changes—particularly diaphoresis—become more marked, and urine output declines significantly. Hypotension ensues. In addition, changes in cerebral function become evident consisting chiefly of agitation, disorientation, and memory loss. A common error is to attribute uncooperative behavior to intoxication, drug use, or brain injury when in fact it may be due to cerebral ischemia from blood loss. With class 4 shock (> 40% blood volume loss), profound hypotension is typically accompanied by loss of consciousness and anuria. In this situation, rapid resuscitation with crystalloid and blood products is necessary to prevent imminent death.
With any degree of shock, intravenous balanced salt solution (eg, lactated Ringer solution) should be given rapidly until the signs of shock abate and urine output returns to normal. If shock appears to be due to blood loss, blood transfusion should be given, starting with two units of uncross-matched O-negative blood if cross-matched blood is unavailable. Additional resuscitation with crystalloid and/or blood products is guided by the cause of volume loss and response to fluid administration. Successful resuscitation is indicated by warm, dry, well-perfused skin, a urine output of 30-60 mL/h, and an alert sensorium. Improvement in pH toward normal, correction of lactic acidosis, and minimization of base deficit as measured on an arterial blood gas sample are also indicators of successful resuscitation.
As a general principle, measurements of blood pressure and pulse are less reliable than changes in urine output in assessing the severity of shock. Young and athletic older patients may have compensatory mechanisms which maintain adequate blood pressure even with moderate volume loss. Older patients and those taking cardiac or blood pressure medications often do not exhibit tachycardia even with extreme volume loss. Therefore, a Foley catheter should be inserted into the bladder to monitor urine output in any patient with major injuries or shock. Oliguria is the most reliable sign of moderate shock, and successful resuscitation is indicated by a return of urine output to 0.5-1 mL/kg/h. Absence of oliguria is an unreliable index of the absence of shock if the patient has an osmotic diuresis due to alcohol, glucose, mannitol, or intravenous contrast material.
A patient who is receiving intravenous fluids at a high rate may not exhibit signs of shock even in the setting of ongoing hemorrhage. If a patient continues to require high volumes of fluid after initial resuscitation in order to maintain urine output, mental status, and blood pressure, further investigation must be performed to rule out occult hemorrhage. The patient must be kept recumbent and given reassurance and analgesics as necessary. If opioids are necessary for pain relief, they are best administered intravenously in small doses.
Neurogenic shock is due to the pooling of blood in autonomically denervated venules and small veins and is usually due to spinal cord injury. Neurogenic shock is not caused by an isolated head injury, and in those patients, other causes of shock should be sought. A patient exhibiting signs of neurogenic shock (warm and well-perfused distal extremities in the presence of hypotension) should be given a 2 L crystalloid fluid bolus—followed by an additional bolus if the response is suboptimal. If neurogenic shock persists with fluid resuscitation, phenylephrine or another vasopressor should be given as a drip with the dosage adjusted until the blood pressure is maintained at a satisfactory level. If the patient does not improve quickly, other kinds of shock must be considered. Patients with neurogenic shock may require central venous pressure monitoring to ensure an optimal volume status.
Cardiac compressive shock is caused by compression of the thin-walled chambers of the heart—the atria and the right ventricle—or by compression or distortion of the great veins entering the heart. The usual causes of this type of shock in the trauma patient are pericardial tamponade, tension pneumothorax, massive hemothorax, diaphragmatic rupture with herniation of abdominal contents into the chest, and an elevated diaphragm from massive abdominal hemorrhage. Treatment consists of urgent decompression depending on the specific cause. In severe cases, emergency thoracotomy may be necessary to restore adequate cardiac function.
Cardiogenic shock is caused by decreased myocardial contractility and is most commonly caused by myocardial infarction or arrhythmia. Older trauma patients may develop a myocardial infarction as a complication of their injuries. On occasion an acute myocardial infarction may precede a traumatic event and be a cause of injury or loss of consciousness. Rarely, a severe myocardial contusion may lead to cardiogenic shock. Treatment is supportive, with volume replacement guided by hemodynamic monitoring and administration of inotropic agents to augment cardiac output as necessary to maintain adequate end-organ perfusion. Unfortunately, patients with traumatic injuries are not usually candidates for anticoagulant or lytic therapy, and treatment of their acute myocardial ischemia is sometimes hindered by concerns about bleeding. Echocardiography is helpful for assessment of wall motion abnormalities from severe cardiac contusion.
Immediately after intravenous catheters are placed, a blood sample should be drawn for blood typing and cross-matching. If the patient has a history of renal, hepatic, or cardiac disease or is taking diuretics or anticoagulants, serum electrolytes and coagulation parameters should be measured. In most patients with serious injuries, an arterial blood gas provides rapid data about acidosis and base deficit, both of which are markers of under-resuscitation in addition to oxygenation (Pao2) and ventilation (Paco2). Gross blood in the urine indicates the need for further diagnostic testing with abdominal CT scan or, in selected cases, a cystogram and urethrogram. Patients with obvious severe head injury, where intracranial pressure monitoring may be indicated, should have coagulation studies and a platelet count performed. Measurement of blood alcohol level and urine toxicology screen may be useful in patients with altered mental status.
Radiographic plain films of the chest and pelvis are required in all major injuries. Lateral C-spine films have been supplanted by formal CT scanning of the neck in patients with suspicion of or mechanism for cervical spine injury. Bedside focused assessment with sonography for trauma (FAST) is the preferred triage method for determining the presence of hemoperitoneum in blunt trauma patients or cardiac tamponade in blunt and penetrating trauma patients. The presence of hemoperitoneum in an unstable patient on FAST may be an indication for exploratory laparotomy. Presence of hemoperitoneum in a stable patient or a negative FAST in a patient with abdominal pain is indication for further evaluation with abdominal CT scan.
Patients who have an abnormal chest radiograph with a mechanism for blunt aortic injury should undergo further screening with either helical chest CT done at the time of abdominal imaging or with aortography, if necessary. Cervical spine CT scans should be obtained for patients who are unconscious, have pain in the cervical region, have neurologic deficits, or have painful or distracting injuries. CT scanning of the head should be performed in all patients with loss of consciousness or more serious neurologic impairment. Radiographs of the long bones and noncervical spine can usually be deferred until the more critical injuries of the thorax and abdomen have been delineated and stabilized.
A rapid and complete history and physical examination are essential for patients with serious or multiple injuries. Progressive changes in clinical findings are often the key to correct diagnosis, and negative findings that change to positive may be of great importance in revising an initial clinical evaluation. This is particularly true in the case of abdominal, thoracic, and intracranial injuries, which frequently do not become manifest until hours after the trauma.
Recognition of injury patterns is also important in identifying all injuries. For example, fractures of the calcaneus resulting from a fall from a great height are often associated with central dislocation of the hip and fractures of the spine and of the skull base. A crushed pelvis is often combined with laceration of the posterior urethra or bladder, vagina, or rectum. Crush injuries of the chest are often associated with lacerations or rupture of the spleen, liver, or diaphragm. Penetrating wounds of the chest may involve not only the thoracic contents but also the abdominal viscera. These combinations occur frequently and should always be suspected.
In all cases of patients with multiple injuries, there must be a “captain of the team” who directs the resuscitation, decides which x-rays or special diagnostic tests should be obtained, and establishes priority for care by continuous consultation with other surgical specialists and anesthesiologists. A trauma surgeon or a general surgeon experienced in the care of injured patients usually has this role.
After controlling the airway if necessary, resuscitation and blood volume replacement have first priority. Deepening stupor in patients under observation should arouse suspicion of an expanding intracranial lesion requiring serial neurologic examinations and head CT. Too often, obvious signs of acute alcohol intoxication have been assumed to be the cause of unconsciousness, and intracranial hemorrhage has been overlooked.
Cerebral injuries take precedence in care when there is rapidly deepening coma. Extradural bleeding is a critical emergency, requiring operation for control of bleeding and cerebral decompression. Subdural bleeding may produce a similar emergency. If the patient’s condition permits, CT scanning should be performed for localization of the bleeding within the cranium prior to other operative interventions being initiated. In many cases of combined cerebral and abdominal injury with massive bleeding, laparotomy and craniotomy should be performed simultaneously.
Most urologic injuries are managed at the same time as associated intra-abdominal injuries. Pelvic fractures present special problems and are discussed in Chapter 40. Unless there is associated vascular injury with threatened ischemia of the limb, fractures of the long bones can be splinted and treated on an urgent basis. Open contaminated fractures should be cleansed and debrided as soon as possible. Injuries of the hand run the risk of infection that may result in a lifelong handicap without early effective treatment. Early treatment of the hand at the same time as treatment of any life-threatening injuries avoids infection and preserves the means of livelihood. Tetanus prophylaxis should be given in all instances of open contaminated wounds, puncture wounds, and burns.
Patients with a severe burden of trauma and shock may not be candidates for definitive treatment of all injuries in the immediate setting. Three physiological derangements comprise the “lethal triad” in the trauma literature: hypothermia, acidosis, and coagulopathy. These are boundaries of a patient’s physiologic envelope beyond which the patient will develop irreversible shock and eventual death. Bailing out of the abdomen with damage control maneuvers in a patient headed for the lethal triad is not a sign of defeat; instead it is usually the intelligent option. Early warning signs of physiologic compromise that could lead to the lethal triad are edema of the small bowel, midgut distension, dusky serosal surfaces, tissue that is cool to the touch, noncompliant swollen abdominal wall, diffuse oozing from surgical or raw surfaces, and lack of obvious clot formation. Successful packing relies on clot formation, so employing a strategy of early packing is recommended rather than turning to it as a last resort.
Details of definitive management of injuries are discussed in the sections on trauma that follow and in the various organ system chapters of this book.
[Archives of Surgery Full Text]
All injuries to the neck are potentially life threatening because of the many vital structures in this area. Injuries to the neck are classified as blunt or penetrating, and the treatment is different for each. The patient must be examined closely for associated head and chest injuries. The initial level of consciousness is of paramount importance; progressive depression of the sensorium may signify intracranial bleeding or cerebral ischemia and requires neurosurgical evaluation. Trauma to the base of the neck may lacerate major blood vessels or have associated pneumothorax. Hemorrhage into the pleural cavity may occur suddenly as contained hematomas rupture.
Injuries to the larynx and trachea can be asymptomatic or may cause hoarseness, laryngeal stridor, or dyspnea secondary to airway compression or aspiration of blood. Subcutaneous emphysema in the neck can be present if the wall of the larynx or trachea has been disrupted.
Esophageal injuries are rarely isolated and by themselves may not cause immediate symptoms. Severe chest pain and dysphagia are characteristic of esophageal perforation. Hours later, as mediastinitis develops, progressive sepsis may occur. Mediastinitis results because the deep cervical space is in direct continuity with the mediastinum. Esophageal injuries can be recognized promptly if the surgeon is alert to the possibility and seeks out early diagnosis. Exploration of the neck, radiographic examination of the esophagus with contrast medium, and in selected cases flexible esophagoscopy confirms the diagnosis.
Cervical spine fractures and spinal cord injuries should always be suspected in deceleration injuries or following direct trauma to the neck. If the patient complains of cervical pain or tenderness or if the level of consciousness is depressed, the head and neck should be immobilized (eg, with a rigid cervical collar or sandbags) until cervical radiologic imaging can be performed to rule out a cervical fracture or ligamentous injury.
Injury to the great vessels (subclavian, common carotid, internal carotid, and external carotid arteries; subclavian, internal jugular, and external jugular veins) may follow blunt or penetrating trauma. Fractures of the clavicle or first rib may lacerate the subclavian artery and vein. With vascular injuries, the patient typically presents with visible external blood loss, neck hematoma formation, and in varying degrees of shock. Occasionally, bleeding may be contained and the injury may go undetected for a short time. Auscultation may reveal bruits that suggest arterial injury.
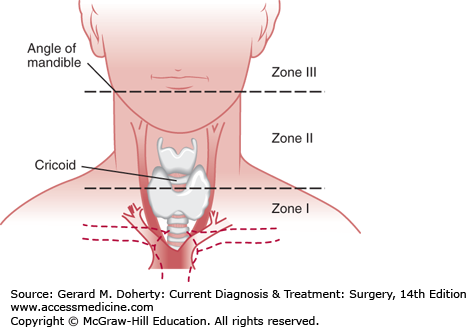
Penetrating injuries of the neck are divided into three anatomic zones (Figure 13–9). Zone I injuries occur at the thoracic outlet, which extends from the level of the cricoid cartilage to the clavicles. Included in this area are the proximal carotid arteries, the subclavian vessels, and the major vessels of the chest. Proximal control of injuries to vascular structures in this zone often requires a thoracotomy or sternotomy. Zone II injuries occur in the area between the cricoid and the angle of the mandible. Injuries here are the easiest to expose and evaluate. Zone III injuries are between the angle of the mandible and the base of the skull. Exposure is much more difficult in this zone and in some cases may require disarticulation of the mandible. High injuries can be inaccessible, and control of hemorrhage may require ligation of major proximal vessels or angiographic embolization.
Penetrating trauma to the posterior neck may injure the vertebral column, the cervical spinal cord, the interosseous portion of the vertebral artery, and the neck musculature. Penetrating trauma to the anterior and lateral neck may injure the larynx, trachea, esophagus, thyroid, carotid arteries, subclavian arteries, jugular veins, subclavian veins, phrenic and vagus nerves, and thoracic duct.
With any penetrating cervical trauma, the likelihood of significant injury is high because there are so many vital structures in such a small space. Any patient with shock, expanding hematoma, or uncontrolled hemorrhage should be taken to the operating room for emergency exploration. The location of the injury suggests which structures may be involved. Vascular injuries at the base of the neck require thoracotomy or sternotomy to obtain proximal control of injured blood vessels before the site of probable injury is exposed. If the patient is stable after resuscitation, additional diagnostic testing may be considered.
Conventional angiography or computed tomography angiography is usually recommended for patients with stable injuries in zones I and III because precise identification of the location and extent of injury may alter the operative approach. If possible, angiography should be performed before exploration of any injury in which blood vessels may be damaged below the level of the cricoid cartilage or above a line connecting the mastoid process with the angle of the jaw. Arterial injuries above this line are practically inaccessible. If injury to the carotid artery at the base of the skull is confirmed by angiography, repair may not be possible and ligation may be required to control bleeding, or angiographic intervention may be required. Injured carotid arteries that have produced a neurologic deficit should be repaired if possible. The morbidity and mortality of patients undergoing carotid artery repair are significantly lower than those who have ligation of the carotid artery (15% vs 50%). Carotid artery ligation is indicated in the patient who presents with uncontrollable hemorrhage or coma with no prograde flow in the carotid artery.
Since exposure of injuries in zone II is relatively easy to obtain, a policy of mandatory exploration was the traditional recommendation for all injuries penetrating the platysma muscle. Although this approach is safe, reliable, and time-tested, studies have demonstrated that a selective approach is as safe provided that diagnostic testing does not detect a major injury and the patient is stable. High-resolution helical CT scanning of the neck can also be used to guide surgical decision making in zone II penetrating injuries. Invasive studies such as endoscopy and conventional angiography can often be eliminated if CT demonstrates a trajectory remote from vital structures such as blood vessels or the aerodigestive tract.
In the absence of an obvious vascular injury on clinical examination, ultrasound with color-flow Doppler has been demonstrated to be reliable in ruling out carotid artery injuries. Computed tomography angiography can also be used in this setting and may offer the advantage of identifying an unsuspected vertebral artery injury. Vertebral artery injuries should also be suspected when bleeding from a posterior or lateral neck wound cannot be controlled by pressure on the carotid artery or when there is bleeding from a posterolateral wound associated with fracture of a cervical transverse process. Flexible or rigid endoscopy can be used to evaluate the trachea and esophagus. A contrast study of the upper esophagus should be performed to identify esophageal injuries that might not be readily apparent on endoscopy. These injuries can be difficult to detect and are occasionally missed on surgical exploration. In either case, repeated, careful examinations should be performed.
The most important injuries resulting from blunt cervical trauma are: (1) cervical fracture, (2) cervical spinal cord injury, (3) vascular injury, and (4) laryngeal and tracheal injury. Radiographic examination of the cervical spine and soft tissues is essential. Careful neurologic examination can differentiate between injuries to the spinal cord, brachial plexus, and brain.
The diagnosis of a cervical spine fracture is reliant on the history, physical examination, and confirmatory studies. The use of plain radiographs has been supplanted by non-contrast computed tomography of the cervical spine. Awake, unimpaired patients with a negative physical examination are unlikely to have a clinically significant cervical spine injury. Obtunded patients with a negative CT scan and no clinical signs of cervical spine injury are also unlikely to have an injury. Following a negative CT scan, the cervical collar is typically removed once the patient is no longer obtunded and the examination can be repeated, or immediately if the patient is likely to remain obtunded/intubated in the ICU. The pediatric patient with an unreliable physical examination often requires additional imaging to clear the spine. Adult patients with a negative CT scan and pain often require flexion and extension views or a MRI to rule out ligamentous injury. Cervical fractures are often managed with external immobilization using rigid collars or a halo/vest apparatus. In some cases, unstable cervical spine fractures require reduction and internal fixation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree