Study
Study type, location and size
Intervention
Inclusion and exclusion
Embryo transfer
Markers of difficult transfer
Outcomes
NOS
Bodri (2008)
Spain, single-centre RCT, n = 330
Trans-abdominal ultrasound versus trans-vaginal
Fresh IVF donor cycles
Day 2 or 3 transfer with soft catheter, full bladder for TA, unclear for TV
Longer, more difficult, repeat transfer or use of dilator. Any amount of blood
Clinical pregnancy rate, ongoing pregnancy rate, miscarriages
7
Drakeley (2008)a
UK-based, single-centre RCT, n = 2,276
Ultrasound-guided versus clinical touch
All IVF and ICSI cycles using fresh and frozen embryos
Variety of soft catheters, ‘comfortably full’ bladder. Day of transfer unclear
Use of outer sheath, stylet or tenaculum
Clinical pregnancy rate
7
Eskander (2008)
Saudi Arabia, single-centre RCT, n = 373
Ultrasound-guided versus clinical touch
Fresh IVF cycles with good-quality embryos
Day 3 transfer with Sydney catheter and full bladder
Blood and mucus on catheter tip
Clinical pregnancy rate
7
Karande (2002)
USA, single-centre, quasi-RCT, n = 251
Cook Echotip™ versus Wallace catheter
Not stated. Fresh, frozen and donor embryo IVF cycles
Day 3 transfer with soft catheter, full bladder and ultrasound guidance
Blood on catheter tip
Clinical pregnancy rate
7
Mansour (1990)
Egypt, single-centre, quasi-RCT, n = 168
Mock transfer prior to IVF cycle
Not stated. Fresh IVF cycles
Day 2 transfer with Wallace, Craft or metal catheter. No ultrasound
Required ‘manipulations and strong push’ or use of the metal catheter
Clinical pregnancy rate
7
Rhodes (2007)
USA, single-centre RCT, n = 99
Cook™ versus Wallace™ catheter
Fresh IVF and ICSI cycles. Less than 40 years old, BMI 20–35, first cycle of IVF
Day 3 transfer with mock transfer at time of transfer. Moderately full bladder/use of ultrasound not clear
‘Tinge’, moderate, or extensive blood on or in the catheter
Clinical pregnancy rate
7
Rhodes (2005)
USA, single-centre, prospective cohort study, n = 205
To determine factors instrumental in ART outcome
Fresh IVF and ICSI cycles. Less than 40 years old, BMI 20–35, first cycle of IVF
Day 3 transfer with soft catheter and mock transfer at time of transfer. Moderately full bladder, ultrasound used in some transfers
‘Tinge’, moderate, or extensive blood on or in the catheter
Clinical pregnancy rate
7
Shaker (1993)b
UK-based, single-centre retrospective cohort study, n = 398
To assess ease of transfer and pregnancy rate
None stated. All cycles included
Unclear
Anything other than a smooth and direct insertion
Clinical pregnancy rate
7
Shaker (1993)b
UK-based, single-centre RCT, n = 120
Sublingual GTN 3 min prior to transfer versus placebo
First cycle of IVF
Transfer with Wallace catheter and empty bladder
Use of outer sheath, tenaculum or uterine sound, or a need to fill the bladder
Clinical pregnancy rate
7
Spandorfer (2003)
USA, single-centre retrospective cohort study, n = 2,263
To identify which factors influence pregnancy outcome
IVF cycles with fresh embryos
Day 3 transfer with Wallace catheter and mock transfer. Ultrasound was not used
Required manipulation, multiple attempts, force, dilatation or resulted in trauma
Clinical pregnancy rate
7
In this chapter, we will review how to perform a uterine evaluation, the role of specific physiological and anatomical variations on the ease of embryo transfer, and how to address the difficult embryo transfer.
How to Perform a Uterine Evaluation
As part of the initial infertility evaluation, anatomical causes of infertility should be addressed with a comprehensive physical exam. Vaginal and/or cervical inspection may reveal structural abnormalities that may suggest the presence of a Müllerian anomaly or cervical stenosis. Bimanual exam may reveal uterine enlargement; an irregular shape, acute cervico-uterine angulation or the lack of uterine mobility may be representative of adhesive disease or the presence of a peritoneal-uterine adhesion [10].
Mid-cycle transvaginal sonography can be used to assess the length of the cervix, the contour of the endometrial stripe, the length of the uterine cavity, the presence of Nabothian cysts, or overt pathology such as a Müllerian anomaly, uterine myoma, or endometrial polyps [11].
A hysterosalpingogram (HSG) allows the assessment of tubal patency and elucidation of submucosal fibroids, uterine polyps, uterine adhesions, and uterine anomalies such as a T-shaped cavity secondary to diethylstilbestrol (DES) exposure or possible Müllerian anomaly, although it is difficult to distinguish a uterine septum from a bicornuate uterus without further evaluation.
It is important to note that an HSG is not a perfect test. Operator errors, such as the presence of air bubbles in the radio-opaque dye, can suggest the presence of an intracavitary lesion, tubal spasm can demonstrate false occlusion of the Fallopian tubes, and tubal fistula or extravasation of the dye into the uterine tissues can suggest false patency [12]. Therefore, this examination cannot be used as the sole evaluation of uterine pathology and should be used in concert with sonographic imaging. In our practice, we find it useful for the reproductive endocrinologist to perform the hysterosalpingogram because it allows the physician to evaluate the difficulty in cannulating the cervix, which is suggestive of the difficulty that may be experienced in the performance of other transcervical procedures, including the embryo transfer [10]. Abnormalities found on HSG usually require further imaging or surgical evaluation (i.e., hysteroscopy or laparoscopy) and, if necessary, surgical correction.
Saline infusion sonohysterography (SIS) is the optimal imaging modality for the evaluation of suspected endometrial polyps and submucosal fibroids. However, in the diagnosis of intrauterine adhesions, it has limited accuracy, similar to that obtained by HSG. Both suffer from a high false-positive diagnosis rate [13]. Of note, three-dimensional SIS may surpass the technical limitations of conventional two-dimensional SIS, allowing for an evaluation of the uterine cavity that is similar to that of a direct hysteroscopic evaluation [14].
If the patient has demonstrable pathology on any of the aforementioned tests or if the study is inconclusive, surgical evaluation may be warranted. Hysteroscopy is the definitive method for evaluation of abnormalities of the endometrial cavity and also, can be used to treat intrauterine (i.e., adhesions, fibroids, polyps) or intracervical (i.e., stenosis) pathology. Laparoscopy allows for the evaluation of peritoneal structures and for the possible treatment of peritoneal/uterine adhesions, which may be causing overt uterine pathology and affecting the contour of the endometrial cavity.
In our program, every patient undergoes a trial transfer, also known as a “uterine sounding” or “uterine mapping”, prior to starting or during controlled ovarian hyperstimulation or at the time of oocyte retrieval. The trial transfer is done using an InsemiTM-Cath (Cook Medical, Spencer, IN, USA) that is attached to a 0.5 cc tuberculin syringe (Fig. 6.1a). The catheter is advanced to the uterine fundus to measure the full length of the uterine cavity and cervical canal. Information garnered regarding the type/size of the speculum required, the direction and curve of the catheter, the length of the cavity, the perceived difficulty of the procedure and the need for ultrasound guidance is recorded (Fig. 6.2). Of note, not every transfer in our program is performed under ultrasound guidance. This information is also available to the physician performing the embryo transfer. In the event that the patient has an interval pregnancy or uterine surgery, the trial transfer procedure is repeated either prior to starting or during COH or at the time of oocyte retrieval. All patients undergoing donor oocyte recipient cycles have a trial transfer performed prior to starting their recipient synchronization cycle.
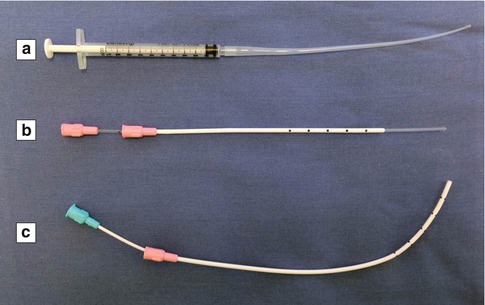
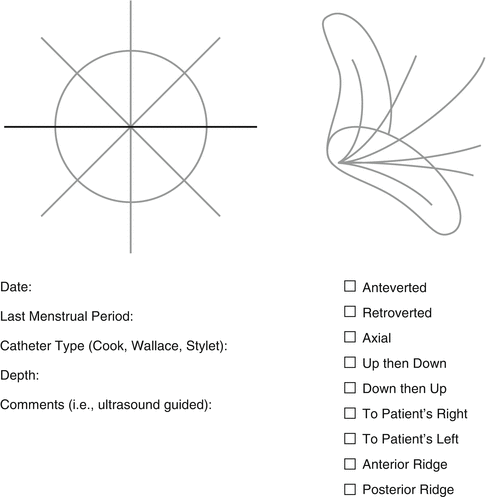

Fig. 6.1
Selection of uterine catheters. (a) InsemiTM-Cath (Cook Medical, Spencer, IN, USA). (b) Wallace® Trial Transfer Catheter (Smiths Medical International Ltd., Hythe, Kent, UK). (c) Wallace® Malleable Stylet (Smiths Medical International Ltd., Hythe, Kent, UK)

Fig. 6.2
Cornell trial transfer record
In all IVF cases, physicians at our center additionally perform a mock embryo transfer immediately before transferring the embryos. The patient is positioned and prepared as described above and a blunt-ended Wallace® Trial Transfer Catheter (Smiths Medical International Ltd., Hythe, Kent, UK) advanced to just past the internal os so as not to disturb the endometrium (Fig. 6.1b). Using the information gained from the trial transfer, the physician does a mock embryo transfer as a means of assessing any potential challenges with the procedure and to also familiarize him- or herself with the patient’s specific uterine alignment and visualize the optimal path of the embryo transfer.
Physiological and Anatomical Variations
Cervical Mucus
As part of the initial infertility physical exam, cervical aberrations may be noted. Cervical factors that may affect a successful embryo transfer may include copious cervical mucus, cervical stenosis, acute cervico-uterine angulation, or anatomical distortion of the cervix to a hard-to-visualize position, such as the anterior vaginal surface. Regardless of the anomaly, passage through the cervix is required for successful transcervical embryo transfer.
It is likely that the easiest cervical challenge to tackle, cervical mucus, if not addressed, has the ability to plug the tip of the ET catheter, thereby making it difficult to gently transfer the embryos to the endometrial cavity, especially since they are transferred in a small volume of culture media. Beyond plugging the ET catheter, it is possible that cervical mucus may be introduced into the uterine cavity along with the catheter and this may affect implantation. In the same regard, if the embryos adhere to the cervical mucus, they may be dragged out of the uterus upon removal of the ET catheter. In fact, the presence of mucus on the catheter tip has been associated with a significantly higher incidence of retained embryos [15, 16]. Along the same lines, blood found outside, but not inside, the transfer catheter after ET is associated with lower rates of embryo implantation and clinical pregnancy although this is likely not related to the same mechanism as cervical mucus and instead, is related to a traumatic ET [17].
In a prospective study by Mansour et al. [18], methylene blue dye was used as a surrogate for culture media in a mock embryo transfer model. As part of their study, the patients underwent mock ET twice, before and after aspiration of the cervical mucus. Their results demonstrated that the dye was extruded at the external os in 57 % of the cases when the cervical mucus was not aspirated compared to 23 % when the mucus was aspirated, a statistically significant difference [18].
In a retrospective analysis of patients undergoing a day 3 ET by a single provider, it was found that by using an embryo afterloading technique, the researchers had a higher clinical pregnancy rate as compared to standard direct ET (52.4 % vs. 34.9 % respectively) [19]. In the embryo afterloading technique, an empty embryo transfer catheter was passed into the lower uterine segment under ultrasound guidance, the inner sheath was slowly removed, and a second inner sheath with the embryos was threaded into the inner sheath into the catheter. The authors postulate that the embryo afterloading technique may result in higher pregnancy rates by preventing mucus contamination/plugging, since there were significantly more transfer catheters with mucus contamination in the direct transfer group (25.58 % vs. 5.95 %) [19].
Furthermore, cervical mucus has been found to be a possible source of bacterial contamination of the endometrial cavity and the embryos [20–22]. In a prospective study of patients undergoing transcervical embryo transfer, microbiological cultures were performed on endocervical swabs and embryo transfer catheter tips. The authors found positive microbial growths from endocervical swabs in 70.9 % of patients and from catheter tips in 49.1 %. The clinical pregnancy rates were 57.1 % in the group of patients without growth and 29.6 % in the group with positive microbial growth from catheter tips [20]. In another study, it was found that the clinical and ongoing pregnancy rates as well as implantation rates were significantly lower in those who had positive versus negative cultures from the tips of the mock embryo transfer catheters immediately prior to embryo transfer (24 % vs. 37 %, 17 % vs. 28 %, and 9 % vs. 16 %, respectively) [21]. The authors reported that the positive cultures were predominantly Escherichia coli (64 %) and Streptococcus species (8 %). Selman et al. [22] conducted a study where separate samples were collected for microbial examination from the following sites: the fundus of the vagina, the cervix, the embryo culture medium prior and post embryo transfer, the tip of the catheter, and the external sheet. All the samples were separately cultured to identify any bacteria or yeast present. They found that the pregnancy rates were significantly lower in only patients testing positive for Entrobacteriaceae and Staphylococcus species [22]. Taken together, the literature supports aspiration and removal of all visible cervical mucus.
Cervical Stenosis
Upon initial inspection of the cervix, it may be noted that the cervical os is either punctate or even difficult to visualize. Cervical stenosis may be congenital, iatrogenic, or secondary to infection, cervical trauma, endometriosis, or postmenopausal atrophy. The classic description of cervical stenosis is a narrowing of the cervical canal to less than 2.5 mm; stenosis of the visible external cervical os has been described as an external os diameter less than 4.5 mm or roughly the size of a cotton-tipped applicator [23, 24]. In patients who have had a laser cone biopsy, there has been a reported incidence of postprocedural cervical stenosis in 17 % of patients within 3–5 years [25]. This is compared to an approximate 10 % risk after a cold knife conization or 4–19 % incidence after a loop electrocautery excision procedure (LEEP) [26–28]. Due to the newly updated American Congress of Obstetricians and Gynecologists cervical cancer screening guidelines and vaccination against HPV, fewer women are having destructive cervical procedures, and as a result, iatrogenic cervical stenosis frequency should diminish. However, since the IVF patient population is an older population, it is possible that many of these women underwent a cervical procedure prior to the changes in the guidelines [29].
Although the cervix may appear stenotic, it is possible that the uterine sound may be able to traverse the os; therefore, it is our program’s policy that the trial transfer be attempted on all visually abnormal cervices in those patients undergoing a future ET. In the event that either the catheter cannot pass through the stenotic external cervical os or the os cannot be clearly identified, the patient should return to the office at the time of menses since the origin of the menstrual blood may help identify the cervical os. In some cases, the patient may warrant surgical intervention either prior to initiation of COH/recipient synchronization or, if absolutely necessary, at the time of oocyte retrieval.
While some authors have advocated for dilation of the cervix at the time of oocyte retrieval given that the patient is already under anesthesia and does not have to come to an additional procedural visit, both Visser et al. [15] and Groutz et al. [30] found that a short interval between dilation and embryo transfer has resulted in poor pregnancy outcomes [15, 30]. This may be due to the fact that the endometrium does not have sufficient time to recover from any trauma, inflammation, or bacterial contamination caused by the dilation [4]. However, Abusheikha et al. [31] evaluated the effects of cervical dilation, performed approximately 2 weeks before embryo transfer and found that of those women who failed to conceive after a prior embryo transfer, nearly 37 % achieved a pregnancy [31]. Therefore, it is advisable to perform cervical dilation prior to initiation of COH/recipient synchronization.
Typically, we manage cervical stenosis with mechanical dilation under monitored anesthesia care (MAC) or “conscious” sedation. As has been previously described, the patient is brought to the operating room with a full bladder, she is placed in dorsal lithotomy position, a speculum is placed, the upper lip of the cervix is grasped with a single-toothed tenaculum, and the cervix is mechanically dilated under transabdominal ultrasound guidance starting with a lacrimal duct probe to ensure that a false passage is not created [32–34]. In the event that the cervix is indistinct or flush against the vagina, a sponge stick can be used to manipulate the uterus with sonographic confirmation. At this point, a tenaculum can be used to grab any remnant of cervical tissue, or as described by Valle et al. [35], the vaginal apex and the cervix can then be probed with a lacrimal dilator; again, transabdominal ultrasound should be used to confirm entry into the endometrial cavity before sequential dilation [35].
Some authors advocate for preoperative prostaglandin (misoprostol) or antiprogestogen (mifepristone) administration in those patients who are anticipated to have a challenging cervical dilation. Misoprostol can be administered orally or vaginally in doses ranging from 200 to 400 mcg. In a systematic review of the literature, Polyzos et al. [36] found that premenopausal women treated with misoprostol had a significantly lower risk for further cervical dilation in the diagnostic setting and a significantly lower risk for cervical laceration in the operative setting when compared with placebo [36]. In addition, the mean cervical width prior to hysteroscopy was significantly higher in premenopausal women treated with misoprostol compared with placebo [36]. In contrast, Cooper et al. [37] also conducted a systematic review of the literature and found that prostaglandin administration conferred no benefit with respect to the pain experienced during cervical dilation and there was only some evidence that misoprostol reduced the amount of force and requirement for dilation of the cervix beyond 5 mm [37]. The authors go on to state that preoperative mifepristone did not have any significant effect on the pain experienced during the procedure or dilation of the cervix [37].
Previous authors have described hysteroscopic endocervical resection or cervical shaving as means of creating a distinct and compatible cervical canal. Wortman and Daggett [38] described a method whereby hysteroscopic endocervical resection was used to create a portal of entry to the endometrial canal for women undergoing a hysteroscopic myomectomy [38]. In their model, they reconfigured the contours of the surgical electrode of a standard hysteroscope. Of the 33 patients that were treated, entry into the endometrial cavity was achieved in all cases without any demonstrable complications [38].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


