Learning Objectives
- Describe the current global burden of malarial disease
- Incorporate knowledge of clinical syndromes associated with malarial disease into diagnosis and initial management
- List medications that may be used in both acute therapy and prevention of malaria with consideration for local resistance patterns
- Outline the social and financial cost of malaria, including the economic impact of clinical disease and control strategies
- Give examples of current strategies for global malaria control, including individual precautions, population-based interventions, and new initiatives in vaccine development
Malaria in History
Malaria, or a disease resembling malaria, has been known for approximately 4,000 years in literature and various other historical sources. The name, stemming from the Italian for “bad air,” has surely influenced human populations for longer than recorded history and continues to cause illness and death to this day. Malaria symptoms were noted in ancient Chinese writings dating back to 2700 bc. There are many Greek references to the disease from the 4th century bc onward. Later, several Roman writers attributed malarial diseases to swampy areas. References to malaria, coming as the result of a bite of certain insects, were discussed in the Susruta, an ancient Sanskrit medical treatise.
The first recorded treatment is from a 2nd-century Chinese text, found in a tomb, known as the “52 Remedies.” Treatment included the Qinghao plant, or Artemisia annua (sweet wormwood), that seemed to reduce fevers associated with the illness. In 1971, the active ingredient, Artemisin, was discovered and named by Chinese scientists. What we now call quinine was used by indigenous peoples in Peru and was brought to light by Spanish Jesuit missionaries during the treatment of the Countess of Chincon. Her fever was reduced and she survived. This treatment became known as cinchona or Peruvian bark. German scientist Hans Andersag discovered chloroquine in 1934, and in 1939 dichlorodiphenyltrichloroethane (DDT) was also discovered in Germany by Othmer Zeidler.
In 1880, Charles Luis Alfonse Laveran (a French Army surgeon) was the first to notice parasites in the blood of patients suffering from this febrile illness that would become known as malaria. The species name Plasmodium was given in 1886, and human parasites falciparum, vivax, malariae, and ovale were named in 1890 and 1893. It was in 1897 that Ronald Ross, a British officer in the Indian medical service, demonstrated that malaria came from infected mosquitoes, subsequently identified as various species of female Anopheles. Italian scientist Battista Grassi is also known to have independently demonstrated this vector at nearly the same time.1
Importance and Distribution
Nearly 3.3 billion people, or approximately half of the world’s population, are at risk for malarial infection each year. In the last 10 years there has been significant growth in the fight against malaria. The World Health Organization (WHO) Malaria Report 2011 suggested that it has observed decreasing total incidence, from between 230 and 400 million/year in 2000, down to 216 million cases/year of malaria in 2010. There was a corresponding decrease in the overall deaths from close to 1 million, down to an estimate of 655,000/year in 2010. Several trends in these data are troublesome though. Most deaths occur in sub-Saharan Africa, and approximately 90% of all-case deaths from malaria are in children under 5 years living in sub-Saharan Africa. This translates to one child dying every minute.2
Human immunodeficiency virus (HIV), tuberculosis, and malaria are the three greatest infectious disease killers at work in the world today. Both tuberculosis and HIV/AIDS caused more deaths (1.8 million people died of AIDS in 2010) than malaria in 2010, but there is a disproportionate effect on pregnant mothers and children under 5, with the highest rates of mortality in the world among these populations in sub-Saharan Africa, as already noted. Due to the investment in malaria control, there is an estimate of 1 million children’s lives saved in sub-Saharan Africa in the past decade.3
There is a general consensus in the international community to try to meet the challenge of overcoming malaria as demonstrated by current global efforts. Part of this desire stems from a previous international campaign that was not successful in eradicating malaria or even limiting it in many parts of the world. This previous campaign ended in the late 1970s. It was not a complete failure, but in many critical areas of the world, especially in sub-Saharan Africa, there was minimal impact in limiting malarial illness and death.4
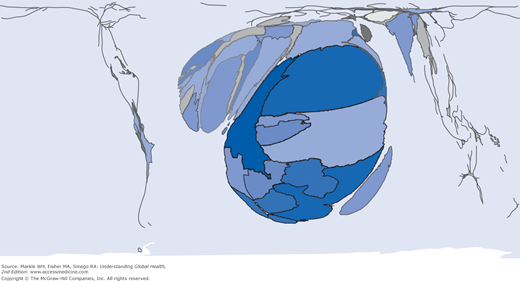
Over the past 10 years, overall incidence of malaria has steadied and then dropped slightly. We seem to be seeing some results of global eradication and control programs with falling total incidence and significant decreases in death rates as well (as much as 50% in some regions). The burden of disease, as demonstrated by Figure 9-1, is still quite concentrated in sub-Saharan Africa, both by total numbers and percentage of cases. During the past decade in Africa, there has been a one-third decrease in the incidence of malaria. At the same time, outside of Africa, 35 of 53 countries affected by malaria have reduced their incidence by 50%. Child mortality from malaria has decreased by 20% over these last 10 years. The Global Malaria Mapper, created by the Medicine for Malaria Venture and the WHO Global Malaria Programme, allows you to access comprehensive worldwide data from the WHO Malaria Report 2012, http://www.worldmalariareport.org/.
Figure 9-1.
World map with incidence of malaria cases by country with countries sized by overall portion of global burden of disease World Mapper Malaria cases. http://www.worldmapper.org/display.php?selected=229.© Copyright SASI Group (University of Sheffield) and Mark Newman (University of Michigan).
Organism, Life Cycle, and Transmission
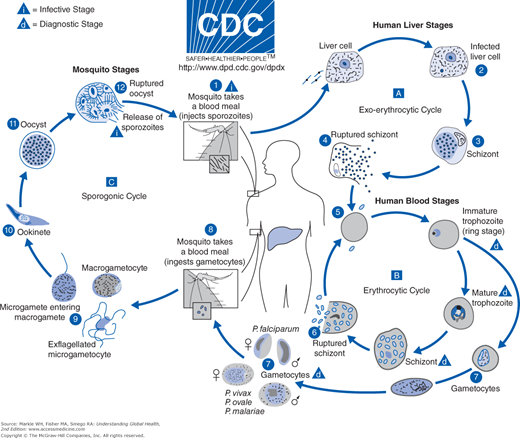
To be successful, malaria parasites must successively infect humans and then female Anopheles mosquitoes. Parasites that began in humans grow and multiply first in liver cells, then into red blood cells, and then into the bloodstream after destroying the red cells in which they live. These daughter parasites, or merozoites, are the particles that go on to invade other red cells and continue the cycle. A female Anopheles mosquito picks up some blood stage parasites, or gametocytes, during a blood meal. Following ingestion, parasites mate and migrate to the salivary glands where a new growth cycle begins. After 10 to 18 days of growth, sporozoites are injected into a new host during a subsequent blood meal, and the human infection begins again by parasitizing liver cells of the human host. Mosquitoes do not suffer from the presence of these parasites but instead act as the vector spreading the disease from human to human (Figure 9-2).
Several conditions must be present for malaria to be transmissible. First there must be climatic and temperature conditions allowing the Anopheles mosquito species to survive and multiply. Second, the proper conditions must exist to allow for immature parasites to complete their growth cycle in the mosquitoes themselves. For example, if the temperature drops below 68°F, this growth cycle cannot occur. (P. vivax can survive at lower temperatures.) Other factors also influence transmission. Transmission will not readily occur at high altitudes, during cooler seasons, in deserts, or in areas where interrupted transmission has occurred through eradication of Plasmodium species. Transmission is much more intense in areas that are warmer and closer to the equator where P. falciparum dominates and where there are year-round conditions for malaria to replicate. There are areas in Europe and North America where Anopheles mosquito species live, but because of eradication via public health measures along with economic development, there are no Plasmodium species to transmit.
Clearly, transmission depends on multiple climatic factors that affect the concentration and survival of mosquito populations. There is seasonal variation; thus transmission is most intense in areas where Anopheles species can survive the longest. The 20 or so Anopheles species also have particular transmission abilities and needs. The important vector species bite at night. All mosquitoes breed in water, but each prefers differing depths of water. Transmission is also more prevalent where the mosquitoes prefer humans rather than other animals. Anopheles gambiae is the dominant vector in Africa and the most efficient vector of all mosquitoes for human disease.
Another critical factor in transmission is human immunity. Moderate host immunity develops in areas of moderate to intense transmission, but it never provides complete protection. Severe disease is less likely with some level of immunity. Thus children are most at risk in high transmission zones; all age groups are at risk in areas with less transmission and lower immunity. Epidemics can occur quickly when some of these factors change suddenly, such as a flood in a normally dry region or an influx of malaria-naive refugees into a malaria-endemic region.
Clinical Features
Malarial illness coincides with the blood stage of infection and may present with a wide range of nonspecific symptoms. Each malaria species does, however, cause some similar clinical features. These occur when red cell schizonts rupture, releasing pyrogens into the bloodstream. Similar features may include the following:
- Fever: Caused by elevated host cytokines released from leukocytes in response to the malarial pyrogens
- Anemia: Caused by both direct hemolysis of red cells and suppression of bone marrow production. This anemia is most pronounced in P. falciparum malaria because red cells of all ages may be infected
- Splenomegaly: Enlarging early in infections by all malaria species. If a patient has had many recurrent infections, the spleen may remain enlarged, leading to secondary hypersplenism
- Jaundice: Hemolysis of red cells may lead to jaundice in all types of malaria. P. falciparum may cause severe jaundice resulting from direct liver involvement.
During well-established infections, the rupture of red cell schizonts may become synchronized. Why this occurs is still unknown. The release of pyrogens during this periodic schizogony leads to regular paroxysms of fever. This observation led to the traditional names of human malaria5:
- Tertian malaria: fever every third day (first day is number 1): P. vivax and P. ovale
- Subtertian (malignant tertian) malaria: fever slightly more frequent than every third day: P. falciparum
- Quartan malaria: fever every fourth day: P. malariae
Clinically, this periodicity often fails to develop and should not be used as a sign of clinical diagnosis. The important implication, however, is that patients with symptomatic or even severe malarial infection may be a febrile at any specific time, and the presence or absence of fever does not necessarily correlate with the severity of disease.
Death from a single acute infection is not an uncommon event. Those who survive develop some level of immunity and have a residual anemia. Recurrent symptomatic events may occur periodically over the course of a year before dying out. This recurrence, called recrudescence, is caused by the persistence of blood forms in small numbers between events.
Infection may be prolonged because illness may not occur for several weeks following initial infection. In the absence of treatment, anemia and hypersplenism may be considerable, and recrudescence of clinical illness may occur for more than 30 years.
P. vivax and P. ovale cause a similar clinical illness. The presence of dormant hypnozoites in the liver may lead to reinvasion of the blood by merozoites, causing periodic clinical relapse for up to 5 years, even if the patient was previously treated with drugs that cleared the blood of parasites.
P. knowlesi is an emerging human pathogen only documented in Southeast Asia, particularly Malaysia, where it may be the most common cause of malarial illness in children. Reports have described severe syndromes resulting from infections with P. knowlesi, but most cases present with a nonspecific febrile illness and a universal thrombocytopenia. They generally follow an uncomplicated course responding to first-line therapies. The most common complication that occurs is respiratory distress and relates directly to the level of parasitemia at presentation.6 Anemia is also a common complication in children.
P. falciparum is set apart from other plasmodia species by its ability to cause severe disease, accounting for the vast majority of more than a million deaths each year. The largest burden of disease falls on children under 5 living in endemic areas because they are exposed recurrently but are still developing immunity. Severe disease may also occur in adults from areas where transmission is unstable in whom very little immunity develops or in travelers to any area who lack or have lost any preexisting immunity. In those with severe disease, the syndromes described next predominate and may occur in isolation or in any combination.
Changes in the level of consciousness, including coma, may occur alone or as a component of other syndromes, including hypoglycemia, acidosis, severe anemia, or as a result of a seizure or a postictal state. If the patient remains unconscious despite attempts to address these complications, he or she may have cerebral malaria. Even in a patient with a parasitemia, another diagnosis may be responsible for the presenting clinical syndrome. Bacterial meningitis, encephalitis, severe pneumonia, or a head injury may also present with changes in level of consciousness.
Cerebral malaria is a diffuse disturbance in cerebral function characterized by a decreased level of consciousness and commonly seizures. The onset may be either gradual or sudden, occasionally preceding other signs of disease, including fever. Limb flaccidity, hypertonicity, posturing, or opisthotonos may accompany coma. Seizure activity may be either generalized or represented by the smallest of repetitive muscle movements. A strict definition includes a patient’s inability to localize painful stimuli and the persistence of coma despite the correction of other potential causes.
A distinctive retinopathy has been recently described. Bedside direct ophthalmoscopy with a short-acting mydriatic may observe areas of whitening of the macula, the extramacular optic fundus, and patchy whitening of small vessels. In an autopsy study, the presence of this retinopathy was the best available clinical predictor of malaria as the cause of death.7 Other less distinctive retinal changes may include white-centered hemorrhages or papilledema.
A detailed pathogenesis of cerebral malaria is not yet clear, but it is likely to include multiple factors. The large burden of sequestered mature parasites in the brain may cause a detrimental metabolic environment to adjacent tissues in which oxygen, glucose, and other nutrients are consumed by parasites that in turn release toxic products, including lactate. This new metabolic environment stimulates the release of host cytokines that further contribute to the development of coma and other complications. Histopathologic changes include a large burden of erythrocytes containing parasites in capillaries and venules of many organs, including the brain. Because more than 90% of patients who recover do not sustain any permanent neurologic sequelae, it is thought that in general this sequestering of erythrocytes is not completely occlusive. However, 5% to 10% of patients are left with some form of neurologic impairment, including hemiparesis, cerebellar ataxia, amnesia, diffuse spasticity, or epilepsy, suggesting that, at least in some cases, the microcirculation may be completed occluded. If antimalarial drugs and supportive care are provided promptly, about 80% of patients with cerebral malaria recover. However, a coma may persist for a few days after the initiation of treatment, especially in adults.
Severe anemia is a common complication, especially in young children living in areas where transmission is high. It may be found incidentally while presenting for unrelated complaints, or it may be the cause of presenting syndromes, including breathlessness, weakness, or a decreased level of consciousness. The clinical presentation may be more affected by the rate of hemoglobin decline than the absolute hemoglobin concentration. Multiple mechanisms for anemia coexist. Large-scale destruction of erythrocytes occurs when schizonts rupture as well as further hemolysis of both parasitized and nonparasitized cells via autoimmune mechanisms. The release of host cytokines leads to a direct suppression of bone marrow production reflected by the absence of a significant reticulocytosis that would be otherwise expected in a hemolytic anemia. Additionally, phagocytosis occurs in the bone marrow and peripheral circulation of both parasitized and apparently uninfected erythrocytes.
A number of factors may contribute to the development of tissue anoxia. The sequestration of parasitized erythrocytes may impair tissue perfusion; combined with anemia, hypovolemia and hypotension leads to localized anaerobic metabolism and the production of lactic acid. Deep breathing initially compensates for the resulting metabolic acidosis but may be insufficient to prevent a falling pH. Dyspnea is therefore the leading presentation of acidosis in children, and if associated with severe anemia or impaired consciousness, mortality may be around 20% to 30%. Mortality may be reduced through rapid fluid volume replacement, using whole blood if necessary.
Hypoglycemia is a common complication of all P. falciparum infections, but children and pregnant women are particularly susceptible. The pathogenesis of hypoglycemia in malaria is also multifactorial but includes the direct suppression of hepatic gluconeogenesis by host cytokines and the consumption of glucose by parasites. Treatment of malaria with quinine or quinidine may cause hypoglycemia because these drugs stimulate the pancreas to release insulin. For this reason, parenteral infusions of these medications should be administered using fluids containing dextrose.
In severe infections with P. falciparum, significant intravascular hemolysis may be associated with hemoglobinuria and acute renal failure. This syndrome was historically known as Blackwater fever. Hemoglobinuria may be precipitated by a drug or dietary factor in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. This type of hemolysis predominantly affects older erythrocytes. Some degree of disseminated intravascular coagulation is also common in P. falciparum infection and may be severe enough to cause bleeding.
Malaria in Pregnancy
Approximately 50 million women live in malaria-endemic areas, and over half of these women live in sub-Saharan Africa. Women in this region have a much higher incidence of severe malaria leading to approximately 10,000 maternal deaths and 200,000 perinatal deaths of children each year.8
Because of poorly understood mechanisms, pregnant women have reduced immune response in pregnancy and thus more difficulty clearing malarial infection. Malaria parasites also sequester and replicate in the placenta and can be passed to the newborn (congenital malaria). Pregnant women have a risk of severe malaria that is three times greater than nonpregnant women and have a higher risk of dying from complications of severe malaria.9
Malarial infection in pregnancy can lead to miscarriage, low-birth-weight (LBW) infants, congenital infections, premature deliveries, perinatal death, and preventable LBW babies.9
LBW associated with malaria in pregnancy accounts for a third of all LBW children delivered annually. Mechanisms of fetal injury associated with severe malaria in pregnancy include placental insufficiency, severe maternal anemia, maternal hypoglycemia, and high output cardiac failure due to severe anemia.10
Edna looked tired. She had made the 5-hour walk to our district mission hospital several times as her pregnant belly grew. Edna’s home was far down the escarpment of the Rift Valley in western Kenya, at least 2,000 feet below our hilltop hospital. I remembered the last agonizing time I had made that hike, with insufficient water and exuberant medical students from America. The moment of realization that home was back at the top of the hill is still a daunting memory. As I strain to hear the baby’s faint heartbeat through my fetoscope, a well-used metal trumpet pressed against my ear, I can feel Edna’s rapid breathing, much faster than I’d expect in a veteran hill climber, even when pregnant. The skin of her ankles admits my soft push, leaving small finger-size dimples. I look at Edna’s face and see small beads of sweat forming on her forehead, her normally pink conjunctiva pale white against her deeply dark skin. Turning to Edna’s chart I notice that although completing 34 weeks of pregnancy so far, she hasn’t been seen in 2 months. This is important because she has missed the second dose of sulfadoxine-pyrimethamine normally given to patients entering their third trimester. Her first dose, given at her first obstetric appointment, is well documented. This widespread WHO initiative, the Intermittent Presumptive Therapy in Pregnancy (IPTp), has had dramatic effects on the incidence of malarial disease in Africa and especially on the complications during pregnancy and health of the newborn. As I wait for confirmation of my clinical suspicion of malaria from the lab, I remember that although Edna survived many bouts of malaria as a young child, developing a partial immunity to the severe effects, her current pregnancy has left her especially vulnerable. Her body, in an immunologic effort not to reject her growing baby, has inadvertently reduced her protection against malaria. She will develop some immunity to the current parasites in her blood, but not in time to prevent the hemolysis that I expect to find reflected in her hemoglobin. Inpatient parenteral therapy will be financially burdensome on this poor farmer. With the assistance of the WHO, the Kenyan Ministry of Health has provided both clear guidelines for treating Edna and access to low cost artemisinin-based combination therapies (ACTs). Artemether-lumefantrine, our first-line oral ACT, is well tolerated and will reduce her parasite load faster than intravenous quinine. Some iron tablets and her daily diet of sukumawiki, a green leafy plant with a name that translates as “pushing the weak,” will gradually raise her hemoglobin in time for her delivery. With relatives close to town, Edna will stay a few days and visit before starting the long walk down to her valley home. Source: Paul Larson, MD |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree