Major Hepatic Resection for Primary and Metastatic Tumors
Sheung Tat Fan
Introduction
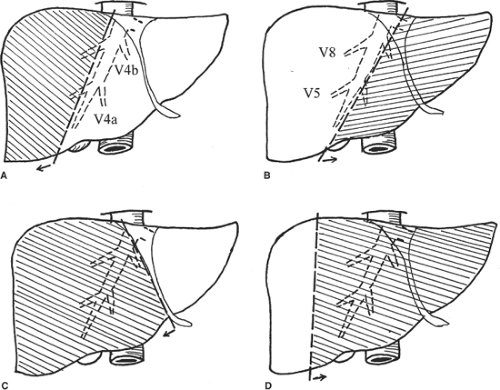
Major hepatectomy is defined as resection of three or more liver segments (Couinaud classification) (Fig. 1). It usually refers to right hepatectomy, left hepatectomy, right lobectomy, or extended left hepatectomy according to the Couinaud nomenclature (Fig. 2). The important anatomic landmark between the right and left livers is the midplane of the liver, in which the middle hepatic vein lies. In <70% of healthy livers, the middle hepatic vein starts in the gallbladder fossa and ends in the inferior vena cava (IVC), either independently or joining the left hepatic vein before it enters the IVC. During the course, the middle hepatic vein receives segments V and VIII hepatic veins on the right side and segments IVa and IVb hepatic veins on the left side (Fig. 2). According to the original Couinaud nomenclature, right lobectomy and extended left hepatectomy mean resection of the right lobe with complete segment IV and left lobe with complete segments V and VIII, respectively. However, for the sake of preserving uninvolved liver tissues, technically, transection of the liver on the left or right side of the middle hepatic vein together with the right or left liver, respectively, is possible, and is called extended right or left hepatectomy in the current usage. Complete resection of segment IV with the right liver is sometimes called right trisegmentectomy and complete resection of segments V and VIII with the left liver is called left trisegmentectomy, according to the Starzl nomenclature. Segment I or caudate lobe could be resected together with the right or left lobe whenever indicated.
Major hepatectomy is indicated for a large tumor (>5 cm) that involves most of the right or left liver. In general, a small tumor should be treated by segmentectomy in order to preserve the liver tissues. Major hepatectomy for a small tumor is indicated only when the tumor is close to the liver hilum, encroaching or involving the right or left portal pedicle, and the liver itself is not cirrhotic. In the past, major hepatectomy was contraindicated if the underlying liver was cirrhotic. Recent attempts in cirrhotic liver with
preserved liver function were, however, successful. Traditionally, major hepatectomy was performed only when the contralateral lobe was not involved by another tumor. However, increasingly, major hepatectomy has been performed with wedge resection or radiofrequency ablation of solitary nodule in the contralateral lobe with satisfactory long-term results. Major hepatectomy is also performed safely today, together with resection of the diaphragm, IVC, right adrenal gland, right kidney, colon, duodenum, or stomach en bloc when the tumor involves such structures.
preserved liver function were, however, successful. Traditionally, major hepatectomy was performed only when the contralateral lobe was not involved by another tumor. However, increasingly, major hepatectomy has been performed with wedge resection or radiofrequency ablation of solitary nodule in the contralateral lobe with satisfactory long-term results. Major hepatectomy is also performed safely today, together with resection of the diaphragm, IVC, right adrenal gland, right kidney, colon, duodenum, or stomach en bloc when the tumor involves such structures.
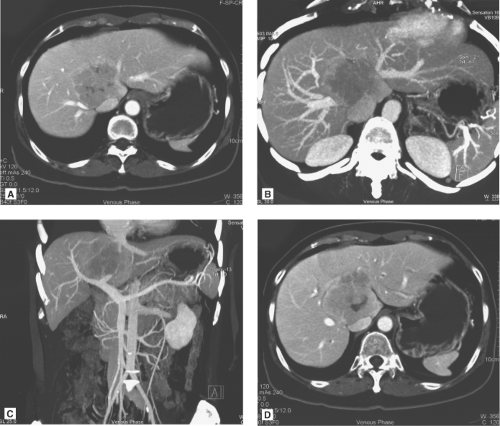
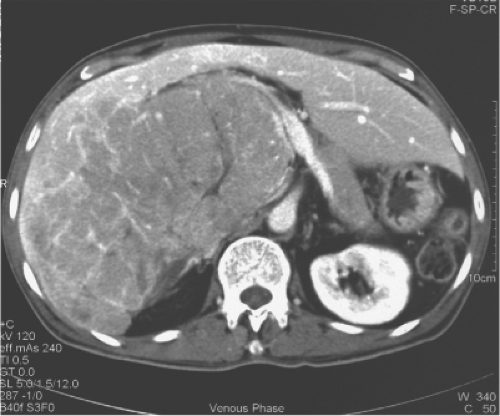
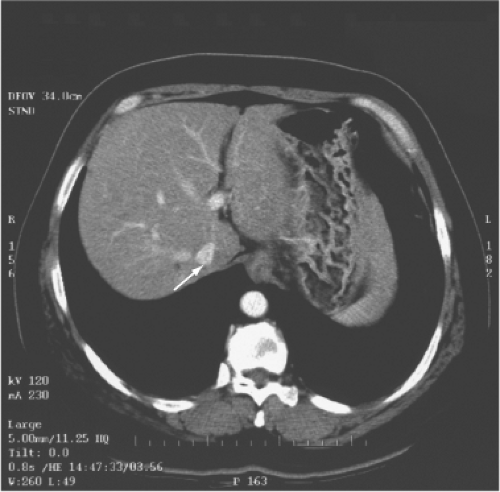
The multidetector computed tomography (CT) scan is the single most reliable imaging modality in deciding on the resectability of the tumor, based on anatomic ground and CT volumetry of the remnant liver. CT scan demonstrates the topographic relationship of the tumor with the middle hepatic vein, right and left portal veins and IVC, and invasion of the tumor into the major vessels and bile duct (Fig. 3). Resectability depends on whether the contralateral hepatic and portal veins (e.g., right hepatic vein in the case of left trisegmentectomy and left hepatic vein in the case of right trisegmentectomy) are intact. Hepatic duct involvement is not a contraindication for major hepatectomy because the hepatic duct could be resected and the biliary tract is reconstructed by a hepaticojejunostomy (Fig. 3). Even major vessels could be resected followed by restoration of continuity by a vein graft obtained directly from the patient or a stored vein graft from a deceased transplant donor.
CT volumetry of the remnant liver is necessary for patients with chronic liver disease. Patients with chronic liver disease and liver remnant <40% of the estimated standard liver volume (as calculated by the Urata formula) may not recover well after major hepatectomy. Right portal vein embolization could be employed to induce right lobe atrophy and left lobe hypertrophy. Liver with chronic hepatitis or mild cirrhosis may undergo hypertrophy in 3 to 6 weeks’ time. However, the tumor may continue to grow during the waiting period. Transarterial oily chemoembolization has been used to control hepatocellular carcinoma (HCC) while waiting. The benefit of portal vein embolization in terms of reduction of operative mortality and prolongation of long-term survival has not been documented by a prospective randomized study. It should not be used in patients with moderate or severe cirrhosis because liver regeneration never occurs and the procedure is potentially harmful to the patient. For patients with healthy liver, a remnant liver volume of 30% of the estimated standard liver volume is sufficient for posthepatectomy survival. Therefore, portal vein embolization is occasionally necessary for patients with healthy liver.
Hepatic angiography is rarely required today. It was performed in the past for showing the arterial anatomy and served as a “road map” for hilar dissection. Now the arterial anatomy can be clearly and accurately shown by CT scan and verified by intraoperative palpation.
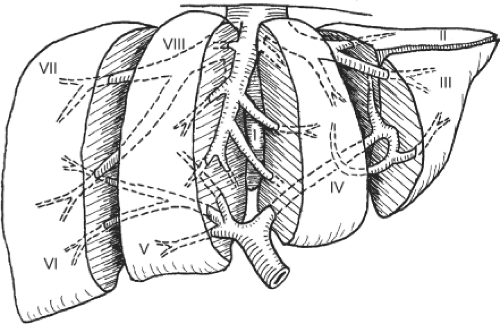 Fig. 1. Schematic diagram showing the liver segments assigned by Couinaud. (Modified from Blumgart LH. Surgery of the Liver and Biliary Tract, 3rd ed. New York: Churchill Livingstone; 1998.) |
Evaluation of liver function is particularly important for patients with HCC because the liver is usually cirrhotic or showing changes of chronic hepatitis. For patients with liver metastases, liver function assessment is also essential because chemotherapeutic agents may induce steatohepatitis, which is frequently used to downsize liver secondaries. Many liver function tests have been proposed for evaluation and selection of patients for hepatectomy. Child-Pugh liver function grading is commonly employed.
In general, only patients with Child-Pugh A liver function are suitable for major hepatectomy. However, among patients with Child-Pugh A liver function, the hospital mortality rate after major hepatectomy is still high. Therefore, in the recent past decades, many quantitative liver function tests were developed.
In general, only patients with Child-Pugh A liver function are suitable for major hepatectomy. However, among patients with Child-Pugh A liver function, the hospital mortality rate after major hepatectomy is still high. Therefore, in the recent past decades, many quantitative liver function tests were developed.
One of the commonly employed tests is the indocyanine green (ICG) clearance test. It was suggested by Japanese researchers to be useful for assessment of liver function and has been confirmed as valuable in quantitating liver function by subsequent studies. We demonstrated that an ICG retention rate of 14% at 15 minutes after intravenous administration is the cutoff point that maximally separates patients with or without hospital mortality after major hepatectomy. The ICG clearance test is probably superior to the Child-Pugh liver function grading because among patients with Child-Pugh A function, the range of ICG retention is quite wide. By multivariate analysis, the ICG clearance is the only test that could predict hospital mortality of cirrhotic patients having major hepatectomy in our center. However, in some patients, the ICG retention rate is high, and other liver function parameters, such as prothrombin time and platelet count, are normal. Such patients may have partial portal vein compression or bile duct obstruction by the tumor because clearance of ICG depends on the portal vein blood flow and bile duct patency (Fig. 4). Before rejecting a patient for hepatectomy based on ICG clearance test alone, the surgeon should view the CT scan carefully for reasons accounting for the high ICG retention rate.
Preoperative assessment of the patients should include screening for comorbid medical illness because comorbid illness was shown to be a major determinant of occurrence of postoperative complications and mortality in recent series of hepatectomy after the patients had been carefully selected on the basis of the ICG clearance test. If present, it should be vigorously treated before surgery. If serious (e.g., recent myocardial infarction, uncorrected uremia), the patients should not be subjected to surgery. Comorbid illness is particularly prevalent in elderly people. Elderly people are at higher risk of major hepatectomy for other reasons: their livers are relatively small because of aging; liver function is suboptimal
because fatty change is not infrequently present; their costal arch is rigid, causing difficulty in exposure of the upper abdomen; and it is difficult to bring down their central venous pressure during liver transection (to facilitate liver transection) without affecting the blood pressure. Therefore, elderly patients are accepted for hepatectomy only if they are free from serious medical diseases.
because fatty change is not infrequently present; their costal arch is rigid, causing difficulty in exposure of the upper abdomen; and it is difficult to bring down their central venous pressure during liver transection (to facilitate liver transection) without affecting the blood pressure. Therefore, elderly patients are accepted for hepatectomy only if they are free from serious medical diseases.
Skin Incision
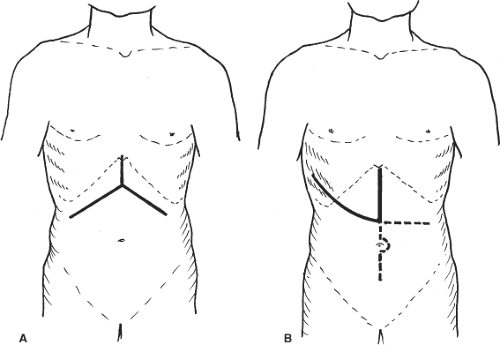
Bilateral subcostal incision with upward midline extension is sufficient for most patients (Fig. 5A). With a self-retaining retractor, the costal arch is pulled up cranially and the entire anterior surface of the liver can be exposed. The midline incision should include excision of the xiphoid process so that after applying the retractor, the suprahepatic IVC could be viewed perpendicularly. Bleeding from the xiphoid bone edge should be controlled by bone wax or argon beam coagulation. Bleeding from the superior epigastric vessels should also be controlled. In case the tumor is located in segment VII or VIII or is bulky, a vertical midline and curved thoracoabdominal extension along the eighth, ninth, or tenth intercostal space (J-shaped incision) could be made (Fig. 5B). This incision provides excellent exposure of the right liver. It allows safe mobilization of the right liver and access to the plane in between the paracaval portion of the caudate lobe and IVC without excessive right liver traction and rotation. Thoracotomy was once thought to be unnecessary and harmful, but recent studies showed that thoracic extension, if indicated, is more advantageous in terms of reducing blood loss and transfusion requirement. For colorectal secondary in the liver and carcinoma of colon or rectum that require synchronous resection, a long midline incision from the xiphoid process to the symphysis pubis could be used.
Right Hepatectomy
The operation starts with cholecystectomy and cannulation of the cystic duct by a fine-bore catheter that will be used for operative cholangiography and detection of bile leakage after liver transection. The right hepatic artery is palpated and its position verified. The hepatoduodenal ligament is incised on the right side to expose the right portal vein. During the procedure, nerve and lymphatic trunks are ligated and divided. The right hepatic artery is usually found between the common hepatic duct and portal vein (Fig. 6). However, the right hepatic artery may cross in front of or lie parallel to the right side of the common hepatic duct. Its position should be detected by palpation before dissection. The dissection of the right hepatic artery should be kept close to the right liver hilum to avoid damage to the segment IV hepatic artery, which may occasionally arise from the right hepatic artery near the right liver hilum. By staying away from the common hepatic duct, arterial supply to the common hepatic duct is preserved.
To avoid inadvertent transection of the main trunk of the proper hepatic artery, a bulldog vascular clamp is applied to the right hepatic artery, and the presence of left hepatic artery pulsation is confirmed by palpation. If Doppler ultrasonography is available, the presence of segments II, III, and IV hepatic artery blood flow could be verified. Then, the bulldog vascular clamp is removed and the right hepatic artery is divided between ligatures.
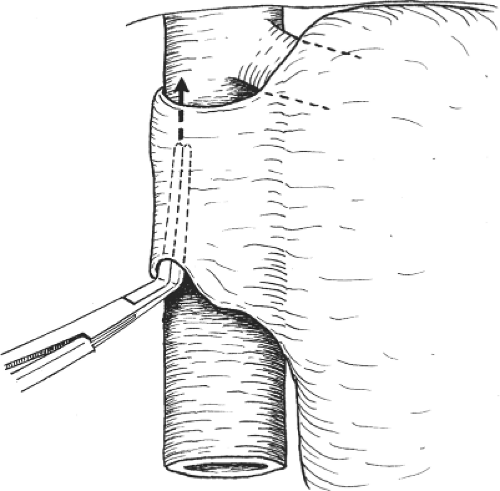
The portal vein is then dissected and the right trunk and bifurcation are exposed. An eyelid retractor lifting up the common hepatic duct will aid dissection of the portal vein (Fig. 6). Several tiny caudate lobe branches arising from the right portal vein have to be ligated and divided to free a sufficiently long length of right portal vein for safe ligation and division. Additional transfixion suture is needed to prevent slippage of ligatures on both divided stumps (Fig. 7). If the stump is short, suturing by 6-0 prolene suture is safer. During the hilar dissection, the dissection on the right hepatic duct is not made unless the confluence of the hepatic ducts is low and obvious. This is because dissection of the right hepatic duct at this time could be difficult, and overzealous dissection of the right hepatic duct may result in removing the hilar plate around the hepatic duct, leading to thinning of the ductal wall and ischemic injury. Closure of the right hepatic duct stump would then be difficult and insecure.
The portal vein is then dissected and the right trunk and bifurcation are exposed. An eyelid retractor lifting up the common hepatic duct will aid dissection of the portal vein (Fig. 6). Several tiny caudate lobe branches arising from the right portal vein have to be ligated and divided to free a sufficiently long length of right portal vein for safe ligation and division. Additional transfixion suture is needed to prevent slippage of ligatures on both divided stumps (Fig. 7). If the stump is short, suturing by 6-0 prolene suture is safer. During the hilar dissection, the dissection on the right hepatic duct is not made unless the confluence of the hepatic ducts is low and obvious. This is because dissection of the right hepatic duct at this time could be difficult, and overzealous dissection of the right hepatic duct may result in removing the hilar plate around the hepatic duct, leading to thinning of the ductal wall and ischemic injury. Closure of the right hepatic duct stump would then be difficult and insecure.
The next step is detachment of the caudate process and paracaval portion of the caudate lobe from the right and anterior surface of the IVC. This step begins with lifting up the caudate process and division of the caudate hepatic vein behind the caudate process. The inferior right hepatic vein, if encountered, is also ligated, transfixed, and divided. Then, the right triangular ligament is divided and the right lobe is mobilized and rotated toward the left side. The right adrenal gland is detached from the liver. Difficulty may be encountered when the adrenal gland is adhered firmly to the liver. If so, the plane between the liver and the adrenal gland is divided by diathermy or scissors, and bilateral hemostasis is made afterward. If the tumor is large and near to the right adrenal gland, it is worthwhile to remove the entire right adrenal gland with the liver.
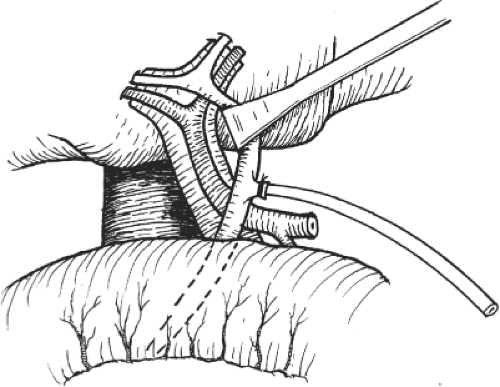 Fig. 6. Right hepatectomy. After cholecystectomy, exposure of the right hepatic artery and portal vein is facilitated by an eyelid retractor. |
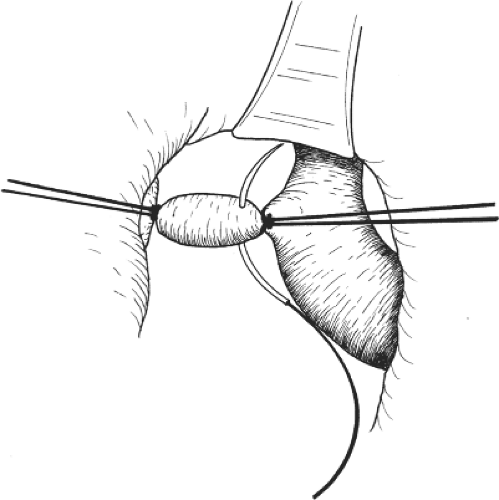 Fig. 7. After ligating the right portal vein, transfixion suture is used to secure the stump before division of the vein. |
On approaching the right side of the IVC, the right IVC ligament will be encountered (Fig. 8). The thickness of the IVC ligament varies. Sometimes, it is composed of liver tissues (Fig. 9). It has to be divided before the right side of the IVC can be exposed or released from the liver. The dissection of the IVC ligament is performed either from the caudal side by the surgeon or from the cranial side by an experienced assistant. The IVC ligament is divided and preferably sutured because it usually contains a vein. Further dissection between the IVC and the liver will expose more fine hepatic veins, which should be ligated and divided. Finally, the right hepatic vein is encircled and divided by an articulated and rotating vascular stapler.
Vascular stapler expedites transection of the hepatic vein. Before its availability, division of the right hepatic vein was sometimes problematic because the right hepatic vein, being short and large, might slip behind the vascular clamps after division and result in massive bleeding from the IVC. During the application of the vascular stapler, the thinner blade of the stapler is usually inserted into the plane between the hepatic vein and IVC instead of the thicker blade because the space for insertion is sometimes limited. However, because the thinner blade is not along the same axis as the instrument, difficulty may be encountered if the tip of the blade impinges on the liver tissues, resulting in liver laceration. A curved or right-angle clamp can be used to grab the thinner blade and guide its insertion through the space (Fig. 10).
After division of the right hepatic vein, further dissection is made until the midline of the anterior surface of the IVC is exposed. A large caudate lobe hepatic vein is preserved in order to maintain liver function, particularly in situations in which the remnant liver is small, cirrhotic, or fatty.
During rotation of the right liver, ischemic damage to the left liver may occur because the inflow and outflow blood vessels
are twisted. Gauze that is inadvertently left in the liver hilum (usually for hemostasis) will aggravate the inflow occlusion. A pack covering the duodenum and stomach may prevent efficient rotation of the right liver. Therefore, all packs and gauzes must be removed during right liver mobilization. The liver must be mobilized in such a way that it rotates to the left subphrenic cavity and is insinuated beneath the incision rather than bringing it out of the wound. The latter maneuver may cause compression injury to the liver by the wound and make mobilization difficult. To allow space for mobilization, the left subcostal wound must be sufficiently long and adequately lifted up by a strong retractor. With all these precautions, ischemic damage to the left liver during rotation from torsion of blood vessels still occurs, although to a minor degree. Therefore, rotation of the right liver has to be intermittent to reduce ischemic damage to the left liver.
are twisted. Gauze that is inadvertently left in the liver hilum (usually for hemostasis) will aggravate the inflow occlusion. A pack covering the duodenum and stomach may prevent efficient rotation of the right liver. Therefore, all packs and gauzes must be removed during right liver mobilization. The liver must be mobilized in such a way that it rotates to the left subphrenic cavity and is insinuated beneath the incision rather than bringing it out of the wound. The latter maneuver may cause compression injury to the liver by the wound and make mobilization difficult. To allow space for mobilization, the left subcostal wound must be sufficiently long and adequately lifted up by a strong retractor. With all these precautions, ischemic damage to the left liver during rotation from torsion of blood vessels still occurs, although to a minor degree. Therefore, rotation of the right liver has to be intermittent to reduce ischemic damage to the left liver.
The vascular demarcation between the right and the left livers is well defined after ligation of the right hepatic artery and the right portal vein. If not, a right anterior hepatic artery or portal vein might have been missed in the hilar dissection. The proposed transection line is marked exactly at the demarcation by electrocautery. On the inferior surface of the liver, the proposed transection line is marked at the tip of the gallbladder fossa in continuity with the line marked on the anterior surface of the liver. The line is then deviated to the left side of the gallbladder fossa and approaches the proposed site of division of the right hepatic duct and the site of division of the right portal vein (Fig. 11). By adopting such transection plane, the middle hepatic vein will be encountered obliquely on its left side rather than longitudinally, as in the case when the transection is done exactly at the Cantlie line or midplane. This is because in, 70% of the cases, the middle hepatic vein originates near the gallbladder fossa in the midplane and if the initial transection is exactly along the midplane, the middle hepatic vein may be divided longitudinally and torrential bleeding will occur.
After identification, the terminal branch of the middle hepatic vein is transected and exposed on its right side by an ultrasonic dissector and traced all the way down to the junction with the IVC or the left hepatic vein (Fig. 12). The exposure of the middle hepatic vein is preferred as it serves as a guide to the transection plane. It must be noted that the transection plane in right hepatectomy is not vertical in relation to the IVC, but oblique (Fig. 13), unless the middle hepatic vein is displaced by a bulky tumor. Without guidance by the middle hepatic vein and adopting a vertical transection plane, the right anterior portal pedicle will be encountered (Fig. 14). The latter situation causes confusion in recognition of anatomy, reduces the tumor-free resection margin (if the tumor is near to the right portal pedicle), increases the transection time, and leaves many ischemic tissues on the right side of the middle hepatic vein. The residual liver tissues on the right side of the middle hepatic vein are not totally ischemic but nourished by backflow of the hepatic vein. An occult tumor focus left in the hypoxic tissues will grow much faster (because of the release of a larger amount of vascular endothelial growth factor) and is difficult to treat by transarterial oily chemoembolization because it is not supplied by the left hepatic artery but from arteries derived from the greater omentum or bowel.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree