51 Lymphomas
Hodgkin’s lymphoma
Pathology
NLPHL accounts for 5% of HL cases and is more common in men.
Investigations and staging
Once the diagnosis has been made on biopsy, further investigations are needed to assess disease activity and the extent of its spread through the lymphoid system or other body sites. This is called staging and is essential for assessing prognosis, with cure rates for localised tumours (stage I or II) being much higher than those for widespread disease (stage IV). The staging of HL is assessed by the Cotswolds modification of the Ann Arbor classification system (Box 51.1). Information about prognostic factors such as mediastinal mass and bulky disease is included in the classification system. The tests required to establish the stage include a complete history, physical examination, FBC, urea and electrolytes (U and Es), chest X-ray and computed tomography (CT). Other useful tests include erythrocyte sedimentation rate (ESR), serum LDH and liver function tests (LFTs). Positron emission tomography (PET) can be used to detect active residual disease.
Box 51.1 Cotswolds modification of the Ann Arbor classification system for Hodgkin’s lymphoma
| Clinical stage | Defining features |
|---|---|
| I | Involvement of a single lymph node region or lymphoid structure |
| II | Involvement of two or more lymph node regions on the same side of the diaphragm |
| III | Involvement of lymph node regions or structures on both sides of the diaphragm: |
| IV | Involvement of extranodal site(s) beyond that designated E |
| Modifying characteristics | |
| A: no symptoms B: fever, drenching sweats, weight loss X: bulky disease >one-third width of the mediastinum >10 cm maximal dimension of nodal mass E: involvement of a single extranodal site, contiguous or proximal to known nodal site CS: clinical stage PS: pathological stage | |
Management
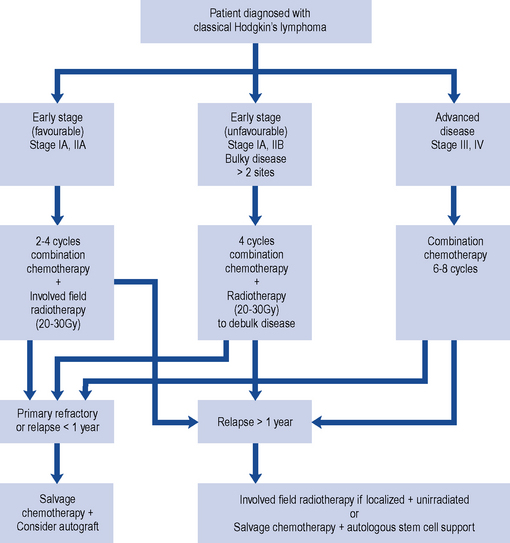
HL is potentially curable and, in general, sensitive to both chemotherapy and radiotherapy; therefore, the two main goals of treatment are to maximise the likelihood of cure whilst minimising the risk of late toxicity such as infertility. Stage of disease is the biggest factor in treatment choice and outcome. The management of classic HL is determined by the stage of the disease, and this is summarised in Fig. 51.1. Localised NLPHL frequently involves one isolated lymph node and tends to be indolent (slow growing). If there are no risk factors, it can be treated with IFRT alone (30 Gy); all other types are treated as advanced (stage III or IV) classic HL.
In Europe, the treatment of classic HL is determined by whether the disease is staged as early favourable disease, early unfavourable disease, advanced disease or relapsed (see Box 51.2).
Box 51.2 Disease staging for Hodgkin’s lymphoma (West of Scotland Blood Cancer Network, 2009)
Early stage
European Organisation for Research and Treatment of Cancer (EORTC) risk factors in localised disease
Early-stage (favourable) disease
The cure rate for patients with stages I and IIA disease is greater than 90%. Patients with stages I and IIA disease may be cured with radiotherapy alone (wide or extended field irradiation). However, due to radiation-related late effects, cardiac toxicity and secondary malignancy and the incidence of relapse (25–30%), most receive combined modality treatment (chemotherapy and radiotherapy). This usually consists of two to four cycles of ABVD (Adriamycin (doxorubicin), bleomycin, vinblastine, dacarbazine) chemotherapy followed by IFRT of 20–30 Gy (Diehl et al., 2004). The aim of chemotherapy is to destroy subclinical disease outside the field of radiotherapy.
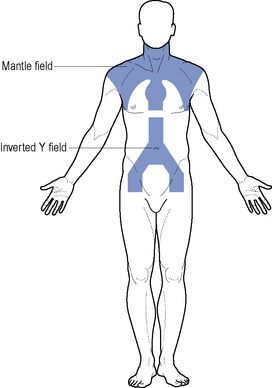
Where disease is confined to above the diaphragm, the mantle field is used (Fig. 51.2). The inverted Y is employed when the disease is confined below the diaphragm. This group of patients have a relapse-free survival rate of 80% at 5–10 years. Patients should be considered for entry into the National Cancer Research Institute (NCRI) 18–30 study or NCRI Lymphoma Groups PET in Hodgkin’s Disease Study.
Advanced disease
Despite advances in HL, 30–40% of patients progress or relapse and respond poorly to salvage chemotherapy. A number of regimens have been investigated to both increase dose intensity and density of treatment, for example, BEACOPP (bleomycin, etoposide, Adriamycin (doxorubicin), cyclophosphamide, vincristine, procarbazine, prednisolone) and escalated BEACOPP (Table 51.1). In a trial comparing escalated BEACOPP, standard BEACOPP and COPP-ABVD, escalated BEACOPP demonstrated increased overall survival compared with the other two treatment arms but at the cost of significant toxicity (Engert et al., 2009). First-line escalated BEACOPP can only be recommended currently as part of a clinical trial. A study to investigate the role of BEACOPP and PET imaging in patients with advanced HL is under way and will close in 2012 (UK Clinical Research Network Study Portfolio, 2010).
Table 51.1 Combination chemotherapy regimens effective in the treatment of Hodgkin’s lymphoma
| Regimen | Dose and route | Frequency |
|---|---|---|
| ABVD (28-day cycle) | ||
| Doxorubicin | 25 mg/m2 i.v. | Days 1 and 15 |
| Bleomycin | 10,000 iu/m2 i.v. | Days 1 and 15 |
| Vinblastine | 6 mg/m2 i.v. | Days 1 and 15 |
| Dacarbazine | 375 mg/m2 i.v. | Days 1 and 15 |
| BEACOPP escalated (21-day cycle)a | ||
| Bleomycin | 10,000 iu/m2 i.v. | Day 8 |
| Etoposide | 200 mg/m2 i.v. | Days 1–3 |
| Adriamycin (doxorubicin) | 35 mg/m2 i.v. | Day 1 |
| Cyclophosphamide | 1250 mg/m2 i.v. | Day 1 |
| Vincristine | 1.4 mg/m2 i.v. (max. 2 mg) | Day 8 |
| Procarbazine | 100 mg/m2 orally | Days 1–7 |
| Prednisolone | 40 mg/m2 orally | Days 1–14 |
| BEACOPP standard dose (21-day cycle)a | ||
| Bleomycin | 10,000 iu/m2 i.v. | Day 8 |
| Etoposide | 100 mg/m2 i.v. | Days 1–3 |
| Adriamycin (doxorubicin) | 25 mg/m2 i.v. | Day 1 |
| Cyclophosphamide | 650 mg/m2 i.v. | Day 1 |
| Vincristine | 1.4 mg/m2 i.v. (max. 2 mg) | Day 8 |
| Procarbazine | 100 mg/m2 orally | Days 1–7 |
| Prednisolone | 40 mg/m2 orally | Days 1–14 |
| ChlVPP | ||
| Chlorambucil | 6 mg/m2 orally | Days 1–14 |
| Vinblastine | 6 mg/m2 i.v. | Days 1 and 8 |
| Procarbazine | 100 mg/m2 orally | Days 1–14 |
| Prednisolone | 40 mg/m2 orally (max 60 mg) | Days 1–14 |
a Escalated BEACOPP and standard BEACOPP have shown activity in Hodgkin’s lymphoma, but their usage is not standard in the UK.
Salvage therapy for relapsed disease
Commonly used chemotherapy salvage regimens are listed in Table 51.2. If relapse occurs less than a year after treatment (early relapse), then high-dose chemotherapy with autologous stem cell support should be considered. A patient who has never achieved complete remission (primary refractory disease) should receive high-dose chemotherapy with autologous stem cell support.
Table 51.2 Salvage chemotherapy regimens effective in the treatment of lymphoma
| Regimen | Dose and route | Frequency |
|---|---|---|
| DHAP | ||
| Cisplatin | 100 mg/m2 i.v. | Days 1 |
| Cytarabine | 2000 mg/m2 i.v. 12 hourly | Day 2 |
| Dexamethasone | 40 mg orally | Days 1–4 |
| ESHAP | ||
| Etoposide | 40 mg/m2 i.v. | Days 1–4 |
| Methylprednisolone | 500 mg/m2 i.v. | Days 1–5 |
| Cytarabine | 2000 mg/m2 i.v. | Day 1 |
| Cisplatin | 25 mg/m2 i.v. | Days 1–4 |
| ICE | ||
| Ifosfamide | 5000 mg/m2 i.v. | Day 2 |
| Carboplatina | AUC 5 i.v. | Day 2 |
| Etoposide | 100 mg/m2 i.v. | Days 1–3 |
| IVE | ||
| Epirubicin | 50 mg/m2 i.v. | Days 1 |
| Etoposide | 200 mg/m2 i.v. | Days 1–3 |
| Ifosfamide | 3000 mg/m2 i.v. | Days 1–3 |
AUC, area under the curve; GFR, glomerular filtration rate.
a Carboplatin dose (mg) = target AUC (mg/mL × min) × (GFR (mL/min) + 25).
High-dose chemotherapy plus autologous stem cell support is associated with a 40–50% 5-year survival rate. However, the significant toxicity of autologous stem cell transplantation means that it should be reserved for patients in whom there is a clear increase in chance of cure. Allogeneic transplant is an option in patients relapsing after autologous transplant (Brusamolino et al., 2009).
New agents
The anti-CD20 antibody rituximab has shown remission in 80% of cases of NLPHL, but due to short follow-up, its use is still considered experimental. Other monoclonal antibodies targeting CD30, which is expressed in the majority of classic HL cases, are being investigated. The most promising of these is a novel immunotoxin conjugate SGN-35 (Younes, 2009).
Gemcitabine has shown activity in relapsed classic HL with response rates up to 79% in a small series of heavily pretreated patients (Ng et al., 2005). Bortezomib and lenalidomide licensed for myeloma are also being investigated in those who have relapsed HL.