Lymphoma
Lester D. R. Thompson, MD
Key Facts
Terminology
Primary thyroid lymphoma comprising heterogeneous group of tumors
Nearly all arise within chronic lymphocytic thyroiditis (80x increased risk)
Clinical Issues
˜ 2-5% of all thyroid gland neoplasms
Mean age: 65 years
Female > > Male (3-7:1)
Patients usually present with stage IE or IIE
Adjuvant chemotherapy and radiation
Mortality is grade and stage dependent (overall, 60% 5-year survival)
Microscopic Pathology
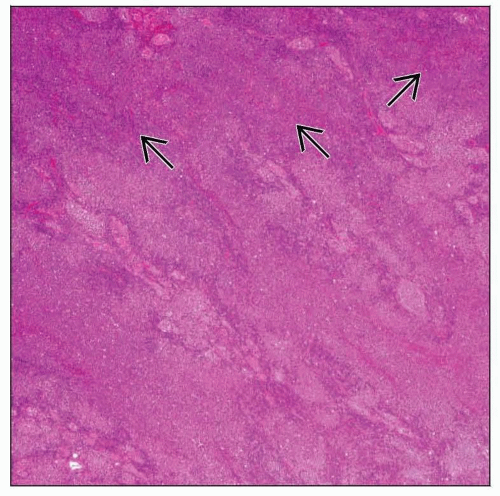
Soft to firm, lobular, bulging cut surface, “fish flesh”
Thyroid gland effaced by atypical lymphoid cells
Lymphoepithelial lesions (LELs) are diagnostic
EMZBCL: Vague nodularity to diffuse effacement
Colonization or follicle lysis by neoplastic B-cells
Atypical small lymphocytes, marginal zone cells, monocytoid B-cells, immunoblasts and centroblast-like cells, plasma cells
DLBCL: Diffuse, large, atypical cells with increased mitotic figures
Ancillary Tests
Usually B-cell immunophenotype (CD20, CD79a)
Keratin highlights lymphoepithelial lesions
Top Differential Diagnoses
Chronic lymphocytic thyroiditis, undifferentiated carcinoma
TERMINOLOGY
Abbreviations
Diffuse large B-cell lymphoma (DLBCL)
Extranodal marginal zone B-cell lymphoma (EMZBCL)
Synonyms
WHO terminology is used
Past lymphoma classifications systems are not to be used
Definitions
Primary lymphoma arising within thyroid gland, usually associated with lymphocytic thyroiditis, comprising a heterogeneous group of tumors
Mucosa-associated lymphoid tissue (MALT) is setting for development of extranodal marginal zone B-cell lymphoma, which may transform into diffuse large B-cell lymphoma
Lack systemic involvement
Regional lymph nodes may occasionally be affected
Rare: Follicular lymphoma (FL), extraosseous (extramedullary) plasmacytoma, Hodgkin lymphoma
ETIOLOGY/PATHOGENESIS
Pathogenesis
Carcinogenesis is multistep, multifactorial process with progressive accumulation of genetic changes
Nearly all lymphomas arise in setting of chronic lymphocytic thyroiditis (Hashimoto disease)
Acquired MALT from autoimmune, immune deficiency or inflammatory process
Nodular or diffuse infiltrate of lymphoid cells, frequently with follicles and germinal centers, and oncocytic metaplasia of thyroid epithelium
Fibrosis and epithelial atrophy supports chronicity
MALT lymphoma shows increased ratio of CD8(+) cells (suppressor/cytotoxic cell) to CD4(+) cells (helper/inducer cell) as compared to lymphocytic thyroiditis
MALT lymphoma cell of origin is from post germinal center, marginal zone B-cells
CLINICAL ISSUES
Epidemiology
Incidence
Uncommon
˜ 2-5% of all thyroid gland neoplasms
˜ 5% of all extranodal lymphomas
EMZBCL: < 2% of all extranodal lymphomas; DLBCL: ˜ 15%
Relative risk of developing a lymphoma is 80x greater in patients with chronic lymphocytic thyroiditis (compared to age- and sex-matched controls)
Age
Mean: 65 years
Wide age range (14-90 years)
Gender
Female > > Male (3-7:1)
Site
Must exclude secondary involvement of thyroid gland
Neck or mediastinal lymph nodes affected by lymphoma directly extending into thyroid gland
Relates to different staging and management
Presentation
Mass or goiter, often with recent rapid enlargement
Causes obstructive symptoms related to compression
Pain
Dysphagia, dyspnea, and hoarseness
˜ 30% of patients
Hypothyroidism (associated with Hashimoto thyroiditis)
Rarely, hyperthyroidism due to follicle destruction
Associated cervical adenopathy in some cases
Choking, coughing, and hemoptysis are uncommon
Symptoms are usually present for short duration
EMZBCL: Mean: 6-12 months
DLBCL: Mean: 4 months
Patients usually present with stage IE or IIE
Patients usually lack B symptoms
Fever, profound night sweats, weight loss, anorexia
Laboratory Tests
Antithyroid serum antibodies usually present
Most patients are euthyroid
Treatment
Options, risks, complications
Surgery for debulking and tissue diagnosis
Radiation may result in mucositis, hypothyroidism, and radiation pneumonitis
Surgical approaches
Obtain tissue for diagnosis: Core needle or partial lobectomy
Previously, surgery included lobectomy or thyroidectomy with lymph node dissection
Surgery is used to debulk or decompress
Adjuvant therapy
Adjuvant chemotherapy and radiation after appropriate classification through needle biopsy
DLBCL: Combined modality therapy
Drugs
Chemotherapy regimens based on histologic type, grade, and stage
EMZBCL: Oral chlorambucil or intravenous chemotherapy
DLBCL: Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy
Radiation
Based on histologic type, grade, and stage
EMZBCL: Single modality radiation therapy (usually up to 40 Gy)
“Involved field only” or “extended field radiotherapy”; latter associated with lower rates of local recurrence or relapse
DLBCL: Hyperfractionated radiation
New modalities
Anti-CD20 therapy and new forms of immunotherapy are experimental but hold promise
Prognosis
Mortality is grade and stage dependent
Overall, approximately 60% 5-year disease-specific survival (DSS), although grade and stage dependent
Stage IE or IIE, low-grade histology: > 95% 5-year DSS
Stage IE or IIE, DLBCL: 50-70% 5-yr DSS
Stage IVE: ˜ 30% 5-yr DSS
Poor prognostic features include
Age > 65 years
Male
High stage (IIIE, IVE)
Dysphagia (vocal cord paralysis)
Extrathyroidal extension
Tumor histology (DLBCL > FL > EMZBCL)
Diffuse architecture
Vascular invasion
High mitotic rate
Combined conservative treatment: Lower relapse rate, reduced distant recurrence, least side effects
Most patients present at stage IE or IIE (extranodal)
DLBCL: More likely to have stage IIIE or IVE
If disseminated, most frequently involved sites are
Regional (cervical), mediastinal, and abdominal lymph nodes
Less common: Bone marrow, gastrointestinal tract, lung, bladder, and liver
IMAGE FINDINGS
Radiographic Findings
MACROSCOPIC FEATURES
General Features
May affect one or both lobes
Soft to firm, lobular and multinodular appearance
Effacement of normal thyroid
Solid and cystic areas
Cut surface: Bulging, smooth, pale-tan, “fish flesh”
Usually homogeneous or mottled
Extension into perithyroidal soft tissues
Size
Wide variation
Range up to 20 cm
MICROSCOPIC PATHOLOGY
Histologic Features
Nearly constant background of chronic lymphocytic thyroiditis
Effacement of normal thyroid gland parenchyma
Ranges from vague nodularity to diffuse effacement
Extension beyond thyroid gland into fat and skeletal muscle in about 50% of cases
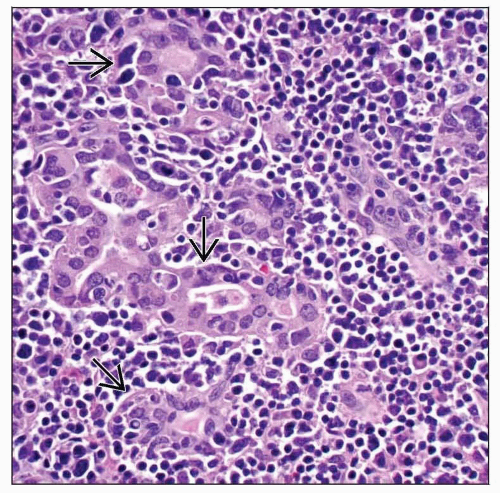
Lymphoepithelial lesions (LELs) are diagnostic
Atypical lymphoid cells infiltrating and destroying thyroid follicular epithelium
2 types
MALT balls: Rounded balls or masses, filling and distending lumen of thyroid follicles
Lymphoepithelial lesion: Single or aggregated lymphocytes within or between follicular epithelial cells
Lymph-vascular invasion common in high-grade tumors
Atrophy of residual thyroid parenchyma and fibrosis
Uninvolved thyroid parenchyma: May have adenomatoid nodules, adenomas, or foci of carcinoma (papillary > > > follicular > medullary)
Vast majority are B-cell lymphomas: EMZBCL and DLBCL with transitions between the two
Single or multifocal areas of large cell transformation adjacent to low-grade component
Extranodal Marginal Zone B-Cell Lymphoma
Extranodal marginal zone B-cell lymphoma (EMZBCL) of mucosa-associated lymphoid tissue (MALT) ± large cell component
Low-grade tumor by definition
Composed of heterogeneous population of B-cells
Vague nodularity to diffuse effacement
Single or multifocal zones of large cell transformation
Transition from low- to high-grade morphology is easy to identify in most cases
20-30% of all thyroid gland lymphomas
Background of chronic lymphocytic thyroiditis in almost all cases
Reactive germinal centers, ± follicle colonization, are invariably present
Colonization or follicle lysis by neoplastic B-cells
These cells yield darker zone within follicles on low power
Follicular architecture may mimic follicle center cell lymphoma
Heterogeneous B-cells include
Atypical small lymphocytes, marginal zone (centrocyte-like) small cleaved cells, monocytoid B-cells, scattered large immunoblasts and centroblast-like cells, and plasma cells
Monocytoid B-cells are monotonous population of atypical lymphoid cells with abundant, pale cytoplasm with lobulated or kidney-shaped nuclei
Small collections of monocytoid cells can be seen
Dutcher bodies and Russell bodies easily identified
Cytoplasmic immunoglobulin (“Mott cells”) and striking plasmacytoid differentiation may simulate plasmacytoma
Crystal-storing histiocytes may be seen
LELs easily identified
Keratin(s) highlights LELs
Increased proliferation index usually within germinal center regions
Infrequently, concurrent disease of gastrointestinal tract, salivary gland, orbit, lung, skin, or breast
Diffuse Large B-Cell Lymphoma
Diffuse, large, atypical cells with increased mitotic figures suggests transformation into diffuse large B-cell lymphoma
60-70% of all thyroid gland lymphomas
Perithyroidal extension into fat or skeletal muscle
Vascular invasion is often seen
Sheets of large, atypical lymphoid cells destroying the thyroid parenchyma
Transitions between EMZBCL and DLBCL are common
However, may occur in absence of low-grade areas
Large cells have spectrum of cytologic features
Centroblasts, immunoblasts, monocytoid B-cells, and plasmacytoid cells
Focal Reed-Sternberg-like cells can be seen
Burkitt-like growth with brisk mitotic activity, apoptosis, “starry sky” pattern
Atrophy of residual thyroid parenchyma and fibrosis are often noted
Extramedullary Plasmacytoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree