KEY CONCEPTS
![]() Lung cancer is the leading cause of cancer deaths in both men and women in the United States. The overall 5-year survival rate for all types of lung cancer is about 15%.
Lung cancer is the leading cause of cancer deaths in both men and women in the United States. The overall 5-year survival rate for all types of lung cancer is about 15%.
![]() Cigarette smoking is responsible for most lung cancers. Smoking cessation should be encouraged, particularly in those receiving curative treatment (i.e., stages I to IIIA non–small cell lung cancer [NSCLC] and limited-stage small cell lung cancer [SCLC]).
Cigarette smoking is responsible for most lung cancers. Smoking cessation should be encouraged, particularly in those receiving curative treatment (i.e., stages I to IIIA non–small cell lung cancer [NSCLC] and limited-stage small cell lung cancer [SCLC]).
![]() NSCLC is diagnosed in most (∼80%) lung cancer patients. NSCLC typically has a slower growth rate and doubling time than SCLC.
NSCLC is diagnosed in most (∼80%) lung cancer patients. NSCLC typically has a slower growth rate and doubling time than SCLC.
![]() Screening test is currently recommended to identify lung cancer in high-risk individuals. However, several studies are evaluating the optimal frequency and duration, as well as the impact of false-positive tests.
Screening test is currently recommended to identify lung cancer in high-risk individuals. However, several studies are evaluating the optimal frequency and duration, as well as the impact of false-positive tests.
![]() Treatment decisions are guided by the stage of disease, which is characterized by tumor size and spread. Patient-specific factors (i.e., performance status, comorbid conditions, etc.) must also be considered when developing a treatment plan.
Treatment decisions are guided by the stage of disease, which is characterized by tumor size and spread. Patient-specific factors (i.e., performance status, comorbid conditions, etc.) must also be considered when developing a treatment plan.
![]() The treatment goals in lung cancer are cure (early stage disease), prolongation of survival, and maintenance or improvement of quality of life through alleviation of symptoms.
The treatment goals in lung cancer are cure (early stage disease), prolongation of survival, and maintenance or improvement of quality of life through alleviation of symptoms.
![]() Early stage lung cancer has the highest cure rates when surgical resection of the tumor is used with or without chemotherapy for NSCLC and chemoradiotherapy for SCLC.
Early stage lung cancer has the highest cure rates when surgical resection of the tumor is used with or without chemotherapy for NSCLC and chemoradiotherapy for SCLC.
![]() Advanced-stage lung cancer is primarily treated with systemic therapy. Doublet chemotherapy regimens are superior in response to single-agent regimens and should be used when the patient can tolerate the associated toxicity. Platinum-containing doublets are first-line treatment in most cases of NSCLC and SCLC.
Advanced-stage lung cancer is primarily treated with systemic therapy. Doublet chemotherapy regimens are superior in response to single-agent regimens and should be used when the patient can tolerate the associated toxicity. Platinum-containing doublets are first-line treatment in most cases of NSCLC and SCLC.
![]() Optimal patient care needs to include prevention and treatment of adverse events from chemotherapy. Adverse events may cause delays in chemotherapy administration, increase morbidity, and contribute to treatment failure.
Optimal patient care needs to include prevention and treatment of adverse events from chemotherapy. Adverse events may cause delays in chemotherapy administration, increase morbidity, and contribute to treatment failure.
Lung cancer is a major cause of morbidity and mortality. It has reached epidemic proportions in many industrialized countries and is the most frequently fatal malignancy in the world. It is estimated that 228,190 new cases of lung cancer were diagnosed in the United States in 2013.1 Despite major advances in the understanding and management of lung cancer, the overall 5-year survival rate for all types of lung cancer remains a dismal 16%. In the United States, lung cancer accounts for about 14% of all newly diagnosed cancer in adults.1 ![]() It remains the leading cause of cancer death in both adult men and women, with about 159,480 deaths in 2013.1 The incidence and death rate caused by lung cancer are declining, which has been attributed to decreased tobacco use over the last 50 years. In comparison to whites, the incidence and mortality of lung cancer is greater in African American men and slightly lower in African American women.1
It remains the leading cause of cancer death in both adult men and women, with about 159,480 deaths in 2013.1 The incidence and death rate caused by lung cancer are declining, which has been attributed to decreased tobacco use over the last 50 years. In comparison to whites, the incidence and mortality of lung cancer is greater in African American men and slightly lower in African American women.1
The incidence of lung cancer increases with age, with about two thirds of cases diagnosed between 60 and 79 years.1 Early lung cancer screening studies failed to demonstrate a survival advantage, but in November 2010, the largest trial of its kind, the National Lung Screening Trial, demonstrated a 20% reduction in the relative risk of death from lung cancer in moderate- to high-risk individuals (95% confidence interval [CI], 6.8 to 26.7; P = 0.004). Among subjects enrolled in lung cancer screening trials, the rate of malignancy in the pulmonary nodule detected on low-dose chest computed tomography (CT) scan is low, and surgical procedures are not without risk. Consequently, patients who receive scans as part of lung cancer screening or for another purpose should have other criteria or tests done before considering a biopsy to evaluate for malignant pathology.2
Patients with lung cancer may undergo surgery, chemotherapy, radiation, or multimodality therapy, depending on the histologic type of the tumor, its size and location, and the presence of metastases at diagnosis.3 Two leading oncology groups representing leading clinicians in the United States have published clinical practice guidelines for the treatment of lung cancer. The National Comprehensive Cancer Network (NCCN) has developed consensus-based guidelines that provide recommendations regarding the screening, staging, and treatment of both small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC).4,5 The American Society of Clinical Oncology (ASCO) first published evidence-based guidelines regarding the staging and treatment of NSCLC in 1997, which were subsequently updated in 2003; the stage IV NSCLC guideline was updated in 2011.6
ETIOLOGY
Lung carcinomas arise from normal bronchial epithelial cells that have acquired multiple genetic lesions and are capable of expressing a variety of phenotypes.2 Significant advances have been made recently in understanding the molecular genetic changes involved in lung cancer pathogenesis.3 A large variety of molecular lesions result in abrogation of key cellular regulatory and growth control pathways. Activation of a proto-oncogene, inhibition or mutation of tumor suppressor genes, and production of autocrine (self-stimulatory) growth factors contribute to cellular proliferation and malignant transformation.3 Many of these molecular alterations are common to both SCLC and NSCLC, but certain mutations are found more frequently in specific subtypes of lung cancer and offer more targeted interventions to prevent or treat lung cancer. In autocrine loop abnormalities, SCLC frequently overexpresses C-KIT (a protein tyrosine kinase receptor that is specific for stem cell factor [aka, CD117]), whereas NSCLC frequently overexpresses epidermal growth factor receptor (EGFR).7 EGFR inhibitors, such as erlotinib, are used clinically to treat NSCLC and offer a potential method of lung cancer chemoprevention. Crizotinib, a drug that targets the EML4-ALK gene rearrangement protein, demonstrates the importance of this pathway in a subset of adenocarcinoma lung cancer patients.8
![]() Smoking is a major cause of lung cancer, with about 80% of lung cancer deaths in the United States directly attributed to tobacco use. Tobacco smoke contains many substances, including tumor promoters, carcinogens, and cocarcinogens,1,7 which are proven carcinogens. The association between environmental tobacco smoke (ETS; also referred to as passive smoking) and lung cancer risk in nonsmokers is not as clear. Most studies have consistently found that spouses of smokers have higher rates of lung cancer than spouses of nonsmokers (about 25% higher risk). In addition, workplace exposure to environmental smoke increases the risk of lung cancer by about 17%. It is currently estimated that ETS contributes to about 3,000 lung cancers annually. Although many of these studies have methodologic flaws, the data seem consistent and seem to indicate a dose–risk relationship, with no safe level of exposure.9 Smoking cessation is associated with a gradual decrease in the risk, but more than 5 years is necessary before an appreciable decline in risk occurs,1,7 and the risk never returns to that of a nonsmoker. Because of the public health implications, the United States has several, mainly state-led, tobacco control efforts, including antismoking campaigns, increased tobacco taxes, and smoke-free areas in many public areas. Although the prevalence of cigarette smoking has slowly decreased, it remains at about 19% in 2010 and 2011.10
Smoking is a major cause of lung cancer, with about 80% of lung cancer deaths in the United States directly attributed to tobacco use. Tobacco smoke contains many substances, including tumor promoters, carcinogens, and cocarcinogens,1,7 which are proven carcinogens. The association between environmental tobacco smoke (ETS; also referred to as passive smoking) and lung cancer risk in nonsmokers is not as clear. Most studies have consistently found that spouses of smokers have higher rates of lung cancer than spouses of nonsmokers (about 25% higher risk). In addition, workplace exposure to environmental smoke increases the risk of lung cancer by about 17%. It is currently estimated that ETS contributes to about 3,000 lung cancers annually. Although many of these studies have methodologic flaws, the data seem consistent and seem to indicate a dose–risk relationship, with no safe level of exposure.9 Smoking cessation is associated with a gradual decrease in the risk, but more than 5 years is necessary before an appreciable decline in risk occurs,1,7 and the risk never returns to that of a nonsmoker. Because of the public health implications, the United States has several, mainly state-led, tobacco control efforts, including antismoking campaigns, increased tobacco taxes, and smoke-free areas in many public areas. Although the prevalence of cigarette smoking has slowly decreased, it remains at about 19% in 2010 and 2011.10
Although most cases of lung cancer are attributable to cigarette smoking, less than 20% of smokers develop lung cancer, which suggests that other risk factors are relevant. An increased risk of lung cancer has been associated with exposure to other environmental respiratory carcinogens (e.g., asbestos, benzene, and arsenic). Genetic risk factors are also important, with an increased risk of lung cancer observed in those with first-degree relatives diagnosed with the disease. Lung cancer risk is associated with polymorphisms that affect the expression and/or function of enzymes regulating metabolism of tobacco carcinogens, DNA repair, or inflammation. Patients with a history of chronic obstructive airway disease and adults with asthma are at an increased risk for lung cancer.1,7,9 Further studies to better identify which patients are at highest risk of developing lung cancer will be key for new lung cancer screening trials and in chemoprevention trials.
HISTOLOGIC CLASSIFICATION
Before treatment begins, it is critical that an experienced lung cancer pathologist reviews the pathologic material because of the different treatment regimens for NSCLC and SCLC. ![]() NSCLC is diagnosed in most (80%) lung cancer patients. NSCLC typically has a slower growth rate and doubling time than SCLC. The histologic classification of NSCLC is well defined and widely accepted (Table 106-1).11 In the most recent classification, the histologic types, subtypes, and identifiable variants convey information about tumors’ natural behavior and in some cases influence therapeutic decisions.4,5,7,11
NSCLC is diagnosed in most (80%) lung cancer patients. NSCLC typically has a slower growth rate and doubling time than SCLC. The histologic classification of NSCLC is well defined and widely accepted (Table 106-1).11 In the most recent classification, the histologic types, subtypes, and identifiable variants convey information about tumors’ natural behavior and in some cases influence therapeutic decisions.4,5,7,11
TABLE 106-1 Histologic Classification of Non-Small Cell Lung Carcinomas

Four major cell types of carcinomas (squamous cell, adenocarcinoma, large cell, and small cell) account for more than 90% of all lung tumors. Early studies with localized disease demonstrated that radiation could cure small cell histology, while surgery did not. Studies with the other histologic types demonstrated better outcomes with surgery than with radiation; hence, the general classification of SCLC and NSCLC was created. Historically, systemic treatment for metastatic squamous cell, adenocarcinoma, and large cell carcinomas was the same and resulted in a similar overall prognosis, which again supported a general classification of SCLC and NSCLC. Trials over the last decade with newer agents have shown differences in efficacy and toxicity with regard to NSCLC histologic types and, consequently, knowledge concerning the histology is essential to optimize drug therapy.4,5,7
Squamous cell carcinoma was once the most common histology, but it now represents less than 30% of all lung cancers. Squamous cell carcinomas have a much higher incidence in smokers and among males and appear to have a strong dose–response relationship to tobacco exposure. Most of these tumors occur centrally, but the incidence of peripheral presentation is increasing. Studies describing the natural history of lung cancer in the era of screening with low-dose CT (LDCT) scans have revealed a relatively constant tumor volume doubling time (104 to 122 days), while the other histologies indicate that smaller tumors found with a CT scan are more indolent (e.g., doubling times three to four times longer with CT-discovered tumors).12 Squamous cell tumors are slower to metastasize, but they eventually spread to the hilar and mediastinal lymph nodes, liver, adrenal glands, kidneys, bone, and GI tract.3,11
Adenocarcinoma accounts for about one-half of lung cancers and is increasing in frequency. It is the most common histology in nonsmoking lung cancer patients. The natural history of adenocarcinoma in the lung shows that small tumors discovered with CT screening are relatively slow growing and the tumor doubling time increases as they get larger; volume doubling time of tumors discovered with CT screening is about 576 days, while those found with routine care double every 169 days.12 This information is most important when considering screening and the potential for lead time bias. Patients with adenocarcinoma can present with a single nodule, multifocal nodules, or rapidly progressing, bilateral, diffuse processes. This histology is likely to metastasize from a relatively small tumor (often before the diagnosis of the primary tumor) and spread widely to distant sites, including the contralateral lung, liver, bone, adrenal glands, kidneys, and CNS. As a result, adenocarcinoma has a worse prognosis than squamous cell carcinoma, but the prognosis is similar when controlled for stage.3,4
Table 106-1 shows several subclassifications and variants of adenocarcinoma. The importance of these subtypes to treatment is currently limited, but newer targeted therapies may work best in certain subtypes, thus allowing more individualized treatment selection. For example, erlotinib was approved because it was effective in NSCLC, but it appears more effective in adenocarcinoma, particularly those with a mutation in the EGFR. The 3% to 13% of NSCLC tumors that have an EML4-ALK rearrangement is almost exclusively adenocarcinoma, which is important to know for testing and if positive will impact therapy.4,13
Large cell carcinomas are undifferentiated epithelial tumors, which are often a diagnosis of exclusion. These tumors tend to be large and bulky tumors arising in the periphery of the lung, have a propensity to metastasize in a pattern quite similar to adenocarcinomas, and are associated with a similar poor prognosis.3,4
SCLCs account for about 15% of all lung tumors. Nearly all SCLCs are immunoreactive for keratin, epithelial membrane antigen, and thyroid transcription factor 1, and many stain positively for markers of neuroendocrine differentiation. They are distinguished by a proliferation of neoplastic cells with round to oval nuclei. These tumors occur in both the major bronchi and the periphery of the lung. SCLC is a very aggressive and rapidly growing tumor, with about 60% to 70% of patients initially presenting with disseminated disease outside of the hemithorax. These tumors commonly express neuroendocrine differentiation, which may account for some of the paraneoplastic syndromes frequently associated with this disease. SCLC secretes gastrin-releasing peptide that acts as an autocrine growth factor. Secretion of other peptide hormones, cytogenetic abnormalities, and amplification and increased expression of oncogenes are also common. This disease has a propensity to metastasize to the lymph nodes, opposite lung, liver, adrenal glands and other endocrine organs, bone, bone marrow, and CNS.3,5
Lung can exhibit more than one histologic cell type (e.g., adenosquamous), which may impact therapy. Patients can also occasionally have multiple lung nodules arising in different lobes or the contralateral lung. They can be the same or different histology. This is referred to as synchronous tumors, and the nodules may be of similar or different cell types. This usually worsens the patient’s overall prognosis.3
CLINICAL PRESENTATION
At the time of diagnosis, 15% of lung cancers are localized, 22% have regional spread, and 56% have distant metastases (the remaining were not staged).1 Location and extent of the tumor determine the presenting signs and symptoms. A lesion in the central portion of the bronchial tree is more likely to cause symptoms at an earlier stage as compared with a lesion in the periphery of the lung, which may remain asymptomatic until the lesion is large or has spread to other areas. The most common initial signs and symptoms include cough, dyspnea, and chest pain or discomfort, with or without hemoptysis.3 Unfortunately, many patients with lung cancer also have chronic pulmonary and/or cardiovascular diseases (usually related to smoking), and such symptoms may go unnoticed or be attributed to the concomitant disease. Many patients also exhibit systemic symptoms of malignancy such as anorexia, weight loss, and fatigue. Disseminated disease can cause extrapulmonary signs and symptoms such as neurologic deficits resulting from CNS metastases, bone pain or pathological fractures secondary to bone metastases, or liver dysfunction resulting from tumor involvement in the liver.3
CLINICAL PRESENTATION Lung Cancer
Paraneoplastic syndromes are signs and symptoms that occur at sites away from the primary tumor or its metastases and are not associated with direct tumor involvement. They may be caused by the production of biologically active substances (e.g., peptide hormones) or antibodies, or by other undefined mechanisms. Paraneoplastic syndromes occur more frequently with lung cancer than with any other tumor, and more frequently with SCLC than with NSCLC. These syndromes may be the first signs of a tumor and may prompt the search for an underlying malignancy.3
SCREENING AND PREVENTION
![]() Most lung cancer patients are diagnosed with advanced disease, which is a key factor in the poor prognosis associated with this disease. Surgery (NSCLC) and radiation (SCLC) are the most effective treatment modalities, which generally limit curative intent to patients diagnosed at an early clinical stage.3–5 Therefore, it is important to diagnose lung cancer earlier, which implies a potential improvement with screening. Several screening techniques, including chest x-ray, CT, and positron emission tomography (PET), scanning have been investigated to detect lung cancer at an earlier stage. The mortality results from screening with a chest x-ray have been negative, but positive results for LDCT scans to screen for lung cancer have been reported. A recent systematic review of the potential benefit and harm from LDCT screening was reported with accompanying recommendations.14 The largest and only positive study was known as the National Lung Cancer Screening trial that enrolled more than 54,000 high-risk smokers. The study reported a decrease in overall (7% vs. 7.5%) and lung cancer–specific (1.3% vs. 1.7%) mortality with LDCT versus control, respectively. The resulting recommendation is to offer annual LDCT screening to individuals aged 55 to 74 years with a 30-pack-year history who are still smoking or have quit for less than 15 years. These recommendations come with a few caveats, including the fact that the most important step is for current smokers to quit. The optimal frequency and duration of screening is unknown and the harm from screening, including frequent false-positive findings, is unknown.14 Consequently, patients interested in screening should be enrolled in a clinical trial so answers to these important questions can be answered.
Most lung cancer patients are diagnosed with advanced disease, which is a key factor in the poor prognosis associated with this disease. Surgery (NSCLC) and radiation (SCLC) are the most effective treatment modalities, which generally limit curative intent to patients diagnosed at an early clinical stage.3–5 Therefore, it is important to diagnose lung cancer earlier, which implies a potential improvement with screening. Several screening techniques, including chest x-ray, CT, and positron emission tomography (PET), scanning have been investigated to detect lung cancer at an earlier stage. The mortality results from screening with a chest x-ray have been negative, but positive results for LDCT scans to screen for lung cancer have been reported. A recent systematic review of the potential benefit and harm from LDCT screening was reported with accompanying recommendations.14 The largest and only positive study was known as the National Lung Cancer Screening trial that enrolled more than 54,000 high-risk smokers. The study reported a decrease in overall (7% vs. 7.5%) and lung cancer–specific (1.3% vs. 1.7%) mortality with LDCT versus control, respectively. The resulting recommendation is to offer annual LDCT screening to individuals aged 55 to 74 years with a 30-pack-year history who are still smoking or have quit for less than 15 years. These recommendations come with a few caveats, including the fact that the most important step is for current smokers to quit. The optimal frequency and duration of screening is unknown and the harm from screening, including frequent false-positive findings, is unknown.14 Consequently, patients interested in screening should be enrolled in a clinical trial so answers to these important questions can be answered.
The term chemoprevention refers to the use of prophylactic medications to prevent the development of cancer. Many studies of potential chemopreventive agents, including nonsteroidal anti-inflammatory drugs (NSAIDs), retinoids, inhaled glucocorticoids, vitamin E, selenium, and green tea extracts, have been conducted, but none have been successful.15 Large randomized clinical trials have evaluated β-carotene as a lung cancer chemopreventive agent in high-risk patients (older smokers). Rather than prevent lung cancer, the trials clearly show that older people who smoke have a higher risk of developing and dying of lung cancer if they take a β-carotene supplement. Nonsmokers do not appear to have an altered risk of lung cancer with β-carotene consumption.16 The impact of selenium and/or vitamin E supplementation was evaluated in older men as part of a large prostate cancer prevention study (Selenium and Vitamin E Cancer Prevention Trial [SELECT]). Unfortunately, no benefit was seen with selenium or vitamin E supplementation.15,16
Because the net benefit of screening is still being defined and chemoprevention trials have not proven to provide a survival benefit, the current recommendation is to avoid smoking and maintain a healthy diet with high amounts of fruits and vegetables.17
DIAGNOSIS
A patient suspected of having lung cancer should undergo a diagnostic evaluation. Diagnosis of lung cancer requires both visualization of the cancerous lesion and tissue sampling for pathologic assessment. All patients must have a thorough history and physical examination with emphasis on detecting signs and symptoms of the primary tumor, regional spread of the tumor, distant metastases, and paraneoplastic syndromes. The patient’s performance status should be assessed to determine whether or not a patient may be able to tolerate surgery and/or chemotherapy.3–5
Visualization of the suspected tumor provides the clinician with the information necessary to choose the most appropriate sampling technique. Chest radiographs, endobronchial ultrasound, CT scans, and PET scans are among the most valuable diagnostic tests.18 Chest radiography is the primary method of lung cancer detection and may also be used to measure tumor size, establish gross lymph node enlargement, and detect other tumor-related findings, such as pleural effusion, lobar collapse, and metastatic bone involvement of ribs, spine, and shoulders. In addition, CT scans may be helpful in the evaluation of parenchymal lung abnormalities, detection of masses only suspected on the chest radiography, and assessment of mediastinal and hilar lymph nodes. PET scans are more accurate than CT scans in distinguishing malignant from benign lesions, detecting mediastinal lymph node metastases, and identifying metastatic spread. Most recently, the use of integrated CT-PET technology has been reported to improve the diagnostic accuracy in the staging of NSCLC over either CT or PET technology alone.19
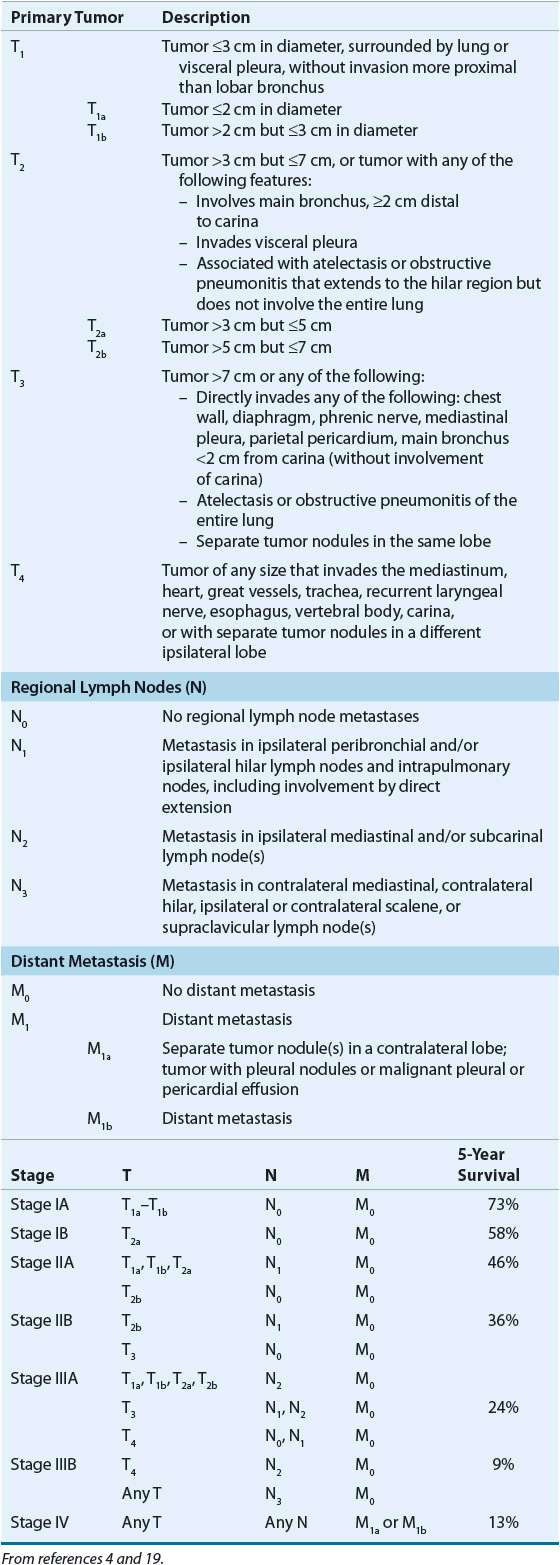
Once the tumor has been located, pathologic examination of tumor tissue is necessary to establish the diagnosis of lung cancer. Tissue is typically obtained through the least invasive method likely to result in an adequate sample; methods include sputum cytology, tumor biopsy by bronchoscopy, mediastinoscopy, percutaneous needle biopsy, or open-lung biopsy. The tissue sample not only confirms malignancy but is also necessary to determine the histology (i.e., squamous cell, adenocarcinoma, large cell, or small cell) and to provide adequate tissue for molecular analysis. Once the diagnosis is established, additional radiologic tests may be required to evaluate lymph nodes and potential metastatic sites for accurate staging. Surgical candidates will have additional sampling of their mediastinal nodes to determine those with stage IIIB (N3) disease (Table 106-2).3–5,19
TABLE 106-2 Tumor (T), Node (N), Metastasis (M) Staging for Non–Small Cell Lung Cancer
STAGING
![]() Once the diagnosis of lung cancer is confirmed, the extent of disease must be determined to estimate prognosis and guide therapy. For NSCLC, tumor growth and spread are staged with the American Joint Committee on Cancer (AJCC) tumor, node, and metastasis (TNM) staging system. SCLC is typically staged with the Veterans Administration Lung Cancer Study Group method.3–5,19
Once the diagnosis of lung cancer is confirmed, the extent of disease must be determined to estimate prognosis and guide therapy. For NSCLC, tumor growth and spread are staged with the American Joint Committee on Cancer (AJCC) tumor, node, and metastasis (TNM) staging system. SCLC is typically staged with the Veterans Administration Lung Cancer Study Group method.3–5,19
Non–Small Cell Lung Cancer
Clinical staging of NSCLC with the TNM system evaluates the size of the tumor, extent of nodal involvement, and presence of metastatic sites. The TNM criteria have recently been updated,20 and went into effect in January 2010.4 The combination of these three evaluations determines the stage. Clinical stages and associated survival rates are described in Table 106-2. For comparison of various therapeutic modalities, a simpler stage grouping system is used in which stage I refers to tumors confined to the lung without lymphatic spread, stage II refers to large tumors with ipsilateral peribronchial or hilar lymph node involvement, stage III includes other lymph node and regional involvement, and stage IV includes tumor with distant metastases. Local disease is associated with the highest cure and survival rates, whereas those with advanced disease have less than a 10% 5-year survival rate.
Small Cell Lung Cancer
The most commonly used system of staging SCLC was developed originally by the Veterans Administration Lung Cancer Study Group. This system categorizes SCLC into two stages: limited and extensive disease. When evidence of the tumor is confined to a single hemithorax and can be encompassed by a single radiation port, the disease is considered limited. Any progression beyond this point is extensive disease. About 60% to 70% of patients initially present with extensive-stage disease. The initial pretreatment evaluation of an SCLC patient should include a medical history, a clinical examination, and laboratory survey, as well as a CT scan of the chest, abdomen, and head. Typically the approach is to identify tumor spread that would demonstrate extensive stage, at which time the workup can stop. For patients without extrathoracic disease identified by these tests, a bone scan and bone marrow biopsy should be performed to confirm limited-stage disease.3,5
TREATMENT
Desired Outcomes
![]() The desired outcomes of lung cancer treatment depend on tumor histology, stage of disease, and patient characteristics such as age, history, and performance status.3 These aspects must be assessed before appropriate treatment can be recommended. In the development of a patient care plan, keep in mind the ultimate goals of therapy. In patients with early stage disease that can tolerate aggressive treatment, a definitive cure is the desired outcome of treatment, although this end point is not always met. With advanced stage disease the desired outcomes of treating lung cancer patients who can tolerate aggressive therapy include prolongation of survival. Regardless of treatment based on survival, all therapies should ultimately improve quality of life through alleviation of symptoms. The goals of treatment must be considered when selecting a therapeutic plan. Delivering aggressive treatment that may prolong survival by a few months but includes a high potential for toxicity that could significantly decrease patient quality of life needs to be considered. Treatment decisions must include both the healthcare team and an informed and well-counseled patient.
The desired outcomes of lung cancer treatment depend on tumor histology, stage of disease, and patient characteristics such as age, history, and performance status.3 These aspects must be assessed before appropriate treatment can be recommended. In the development of a patient care plan, keep in mind the ultimate goals of therapy. In patients with early stage disease that can tolerate aggressive treatment, a definitive cure is the desired outcome of treatment, although this end point is not always met. With advanced stage disease the desired outcomes of treating lung cancer patients who can tolerate aggressive therapy include prolongation of survival. Regardless of treatment based on survival, all therapies should ultimately improve quality of life through alleviation of symptoms. The goals of treatment must be considered when selecting a therapeutic plan. Delivering aggressive treatment that may prolong survival by a few months but includes a high potential for toxicity that could significantly decrease patient quality of life needs to be considered. Treatment decisions must include both the healthcare team and an informed and well-counseled patient.
Non-Small Cell Lung Cancer
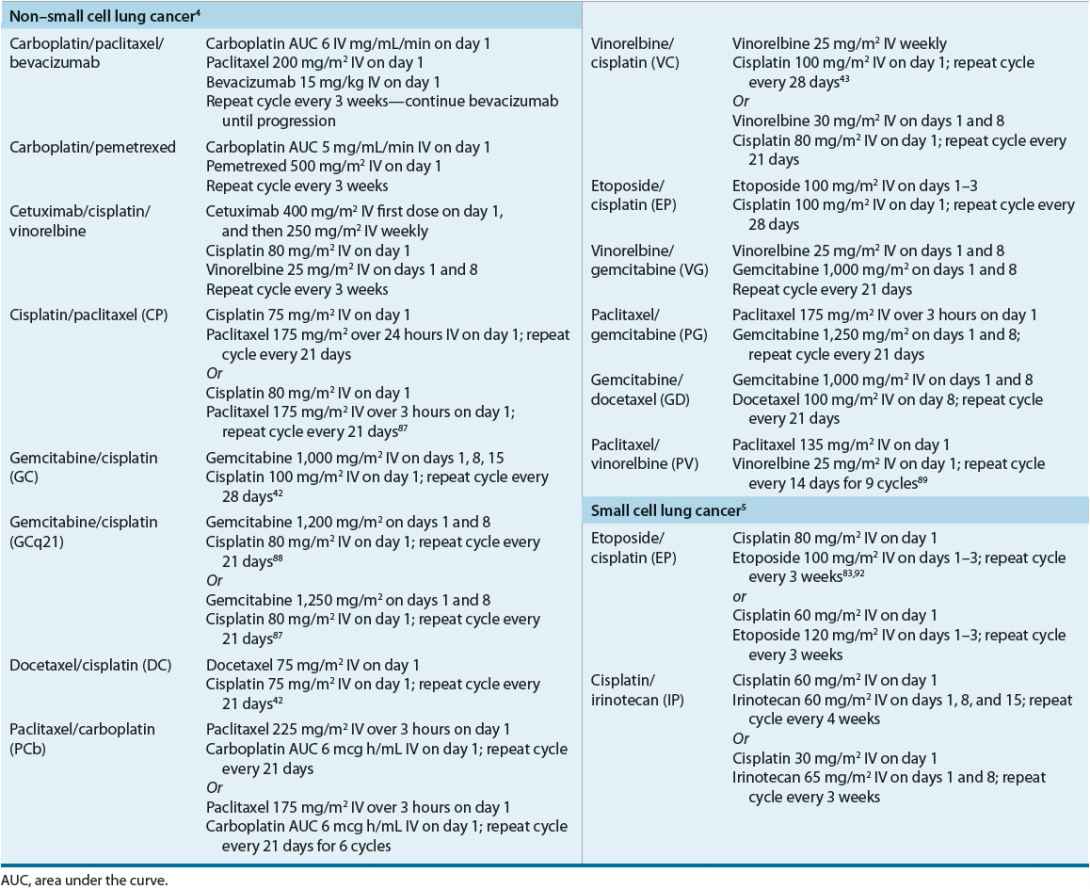
If left untreated, patients with advanced NSCLC will die within 3 to 5 months and those with early stage disease found with routine care will die within 10 to 11 months.12 Surgery, radiation therapy, and systemic therapy with cytotoxic chemotherapy and/or targeted therapies are all used in the management of NSCLC patients. The applications of these treatment modalities are determined by stage and other patient-specific factors (e.g., age, performance status).3,4 Table 106-3 lists commonly used chemotherapy regimens including doses and schedules.4,6
TABLE 106-3 Common Chemotherapy Regimens Used to Treat Lung Cancer
Local Disease
![]() Local disease is associated with a favorable prognosis, and the goal of therapy is cure. Surgery is the mainstay of treatment and may be used alone or in some situations with radiation and/or chemotherapy. Patients who have comorbid conditions preventing them from being surgical candidates can be treated with radiation in place of surgery with curative intent, although the cure rates are lower. Stage IA and IB tumors are treated with surgery alone; when complete resection is achieved, adjuvant therapy is not routinely recommended.21 If surgical margins are positive, re-resection is recommended. Alternatively, patients may receive radiotherapy with or without chemotherapy. Although controversial, patients with IB tumors and high-risk features (poorly differentiated tumors, vascular invasion, wedge resection, minimal margins, tumors >4 cm, or visceral pleural involvement) may also receive adjuvant chemotherapy.3,4,21 Postoperative radiation therapy with older techniques may be detrimental and is not recommended.4,21
Local disease is associated with a favorable prognosis, and the goal of therapy is cure. Surgery is the mainstay of treatment and may be used alone or in some situations with radiation and/or chemotherapy. Patients who have comorbid conditions preventing them from being surgical candidates can be treated with radiation in place of surgery with curative intent, although the cure rates are lower. Stage IA and IB tumors are treated with surgery alone; when complete resection is achieved, adjuvant therapy is not routinely recommended.21 If surgical margins are positive, re-resection is recommended. Alternatively, patients may receive radiotherapy with or without chemotherapy. Although controversial, patients with IB tumors and high-risk features (poorly differentiated tumors, vascular invasion, wedge resection, minimal margins, tumors >4 cm, or visceral pleural involvement) may also receive adjuvant chemotherapy.3,4,21 Postoperative radiation therapy with older techniques may be detrimental and is not recommended.4,21
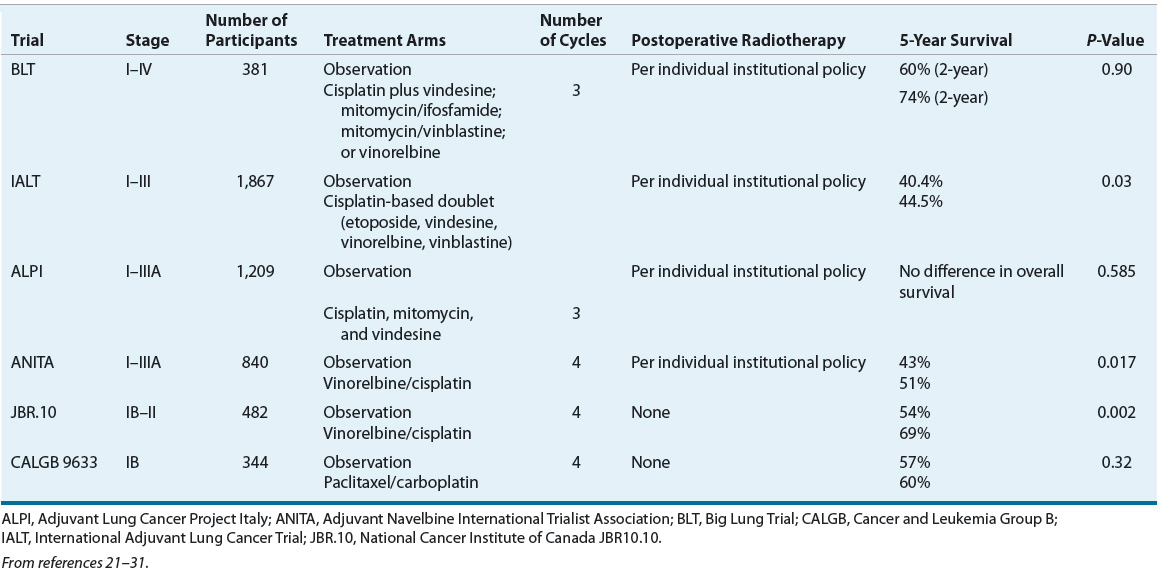
Stage IIA and IIB disease is primarily treated with surgery, which should be followed by adjuvant chemotherapy. The adjuvant treatment regimen of choice is not clear, but the positive clinical trials used platinum-based regimens, with arguably the best data coming from cisplatin–vinorelbine (Table 106-4).4,21,22 The absolute benefit in terms of 5-year overall survival in large randomized trials ranges from no benefit to 15%, with a recent systematic review reporting an absolute difference of 5%.4,21 Adjuvant radiation should be avoided in patients who have complete resection and clean margins because it has not demonstrated to be beneficial and can be detrimental. In those with resected lung cancer and N2 nodal disease, radiation is recommended followed by adjuvant chemotherapy. Radiation, or more commonly chemoradiotherapy, is the treatment of choice for stage II patients who are medically inoperable. Concurrent rather than sequential administration of chemotherapy and radiation therapy is preferred. The chemotherapy portion of concurrent chemoradiotherapy is platinum-based, with the preferred regimen being cisplatin and etoposide.4
TABLE 106-4 Adjuvant Chemotherapy for Early Stage Lung Cancer
Locally Advanced Disease (Stage III)
![]() Patients with more advanced local disease have large tumors, multiple tumors, and/or nodal involvement—particularly mediastinal nodal involvement (N2). Collectively this group of patients is heterogeneous and few well-defined large trials are available to guide treatment. Consequently, treatment is best planned by a multimodality team where individual features and patient input are considered. Optimal outcomes are achieved with multimodality therapy that typically includes systemic chemotherapy. Patients with operable disease should be considered for surgery preceded or followed by systemic chemotherapy. Adjuvant chemotherapy after surgery in selected patients improves overall survival (Table 106-4).4,21–31 The primary adjuvant trials included patients with stage IIIA disease as well as early stage disease; 5-year survival in these studies improved by about 5%. Chemotherapy administration prior to surgery (i.e., neoadjuvant) should also be considered. Hypothetically, it will treat micrometastatic disease prior to surgery and reduce the tumor size making surgery easier and better tolerated. However, it is possible that the tumor will grow and become inoperable during therapy. Two meta-analyses have reported that neoadjuvant chemotherapy improves 5-year survival by about 5% compared with surgery alone.32,33 Although a randomized trial comparing neoadjuvant and adjuvant therapy has not been reported, it appears that both approaches are roughly equivalent and better than surgery alone.
Patients with more advanced local disease have large tumors, multiple tumors, and/or nodal involvement—particularly mediastinal nodal involvement (N2). Collectively this group of patients is heterogeneous and few well-defined large trials are available to guide treatment. Consequently, treatment is best planned by a multimodality team where individual features and patient input are considered. Optimal outcomes are achieved with multimodality therapy that typically includes systemic chemotherapy. Patients with operable disease should be considered for surgery preceded or followed by systemic chemotherapy. Adjuvant chemotherapy after surgery in selected patients improves overall survival (Table 106-4).4,21–31 The primary adjuvant trials included patients with stage IIIA disease as well as early stage disease; 5-year survival in these studies improved by about 5%. Chemotherapy administration prior to surgery (i.e., neoadjuvant) should also be considered. Hypothetically, it will treat micrometastatic disease prior to surgery and reduce the tumor size making surgery easier and better tolerated. However, it is possible that the tumor will grow and become inoperable during therapy. Two meta-analyses have reported that neoadjuvant chemotherapy improves 5-year survival by about 5% compared with surgery alone.32,33 Although a randomized trial comparing neoadjuvant and adjuvant therapy has not been reported, it appears that both approaches are roughly equivalent and better than surgery alone.
Radiation may be given in place of surgery as the local treatment modality combined with chemotherapy. Although a large definitive trial has not been performed, this research question has been evaluated in small randomized trials. The largest trial randomized 333 stage IIIA (N2) patients who responded to three cycles of induction chemotherapy to radiation or surgery. No significant difference in median overall survival (17.5 months vs. 16.3 months for radiation and surgery, respectively) or overall 5-year survival was observed.34 This study suggests that surgery could be avoided by administering chemoradiotherapy, although it does not improve survival. Based on the knowledge that dual-modality therapy was better than a single modality, researchers tested trimodal therapy in small studies. None of the studies to date have demonstrated a survival benefit with chemotherapy, radiation, and surgery so it is considered investigational. The results of a randomized trial (SAKK-16/00) addressing this question are scheduled to be reported in the fall of 2013. It is currently recommended that patients with resectable stage IIIA NSCLC be treated with chemotherapy plus either radiation or surgery, depending on individual patient and tumor features.35
Patients with stage IIIA disease who are not surgical candidates or have a tumor that cannot reasonably be resected and nearly all stage IIIB patients are usually treated with both an active platinum-containing regimen and concurrent radiotherapy. Patients with tumors that cannot fit safely in a radiation port may receive induction chemotherapy followed by chemoradiotherapy. Responding patients may then become surgical candidates. Patients who are not surgical candidates should continue treatment with concurrent chemotherapy and radiation. Patients who are not candidates for radiation are treated like stage IV disease as discussed below.4



