Liver Transplantation
Ronald W. Busuttil
Henrik Petrowsky
Liver transplantation is indicated in patients with life-threatening acute liver failure or irreversible end-stage liver disease (ESLD), which can be caused by various liver diseases (Table 1). But liver transplantation is also indicated in patients with normal liver function who suffer either from malignant liver tumors (hepatocellular carcinoma (HCC), cholangiocarcinoma, metastatic neuroendocrine tumor) or from noncirrhotic liver disease (polcystic liver disease, inherited metabolic disorders).
In chronic, irreversible conditions (cirrhosis), the question arises as to when is the best time for liver transplantation. In this situation, broad indications for liver transplantation are as given:
Hepatic encephalopathy
Hyperbilirubinemia with intractable pruritus
Portal hypertension (varices, upper gastrointestinal (GI) bleeding, ascites, hypersplenism)
Synthetic dysfunction (coagulopathy, hypoalbuminemia)
Poor quality of life (severe fatigue, lethargy)
If two of these five clinical features are present, the patient should be referred for liver transplant evaluation.
Another definitive indication for liver transplantation is HCC, cholangiocarcinoma, and metastatic neuroendocrine tumor. Cirrhotic patients with small HCC within the Milan criteria (single lesion <5 cm or three lesions less than 3 cm each) and without extrahepatic tumor involvement are suitable candidates for liver transplantation. Even if the intrahepatic tumor load is above the Milan criteria but within the UCSF criteria (single lesion <6.5 cm or three lesions total diameter <8 cm), patients are still considered for liver transplantation.
Donor organs are allocated for patients with ESLD according to the severity of their chronic liver disease. In the United States and other countries, the severity of liver disease is determined by the Model of End Stage Liver Disease (MELD) score, which considers the degree of liver and renal failure. The MELD score ranges from 6 in a healthy person to 40 in patients with advanced severe ESLD. The score is composed of serum total bilirubin, serum creatinine, INR. The MELD score is calculated according to the following formula: MELD score 0.957 × ln (creatinine[mg/dL]) + 0.378 × ln (bilirubin [mg/dL]) + 1.12 × ln (INR) + 0.643. Patients without HCC who have a MELD score below 15 should not undergo liver transplantation since there is no benefit from liver transplantation in terms of survival. On the other hand, patients with MELD scores greater than 15 will benefit from transplantation. Patients with HCC or other conditions (hepatopulmonary syndrome, amyloidosis, metabolic diseases) who have a normal or well-compensated liver function will get MELD exception points since the calculated MELD score does not reflect the patient’s medical urgency for liver transplantation.
Acute liver failure in absence of an underlying chronic liver disease is another definitive indication for liver transplantation. Acute liver failure can be caused by various conditions such as drug toxicity (acetaminophen, isoniazid), acute viral hepatitis (hepatitis A, B, E), acute fatty liver of pregnancy, HELLP syndrome, Wilson disease, and mushroom poisoning (α-amanitin). Clinical hallmarks of severe acute liver failure are encephalopathy and coagulopathy. King’s college and Clichy criteria define the urgent need for liver transplantation (Table 2). Patients who define those criteria are urgently listed (status 1) and have the highest priority on the list. If these patients are not urgently transplanted, severe brain edema with herniation and finally brain death can occur. Another condition that requires urgent liver transplantation (status 1) is the primary nonfunction of a transplanted graft.
Contraindications to liver transplantation include active alcohol or drug abuse, HCC beyond Milan and UCSF criteria, severe and irreversible cardiac comorbidities, uncorrectable pulmonary hypertension (mean pulmonary artery pressure >35 mmHg), uncontrollable infection, unstable psychiatric disease, and incompliance.
Table 1 Etiologies of Underlying Liver Disease Requiring Liver Transplantation | |
|---|---|
|
Table 2 Criteria Defining Liver Transplantation for Acute Liver Failure | |||||
|---|---|---|---|---|---|
|
Cadaver Donor Hepatectomy
The donor is placed in the supine position with both arms tucked. The sterile preparation and draping covers the entire chest, abdomen, and proximal thighs to ensure total access to both the thoracic and abdominal cavities. A midline incision is made from the suprasternal notch (two-cavity access) or xiphoid process (one-cavity access) to the symphysis pubis using electrocautery. An extended Balfour self-retaining
retractor with penetrating clamps is used for appropriate abdominal exposure. If the thoracic cavity is opened, a sternal retractor is placed, which improves the exposure especially of both upper abdominal quadrants. Before the start of surgical maneuvers, a thorough exploration of the liver and the abdominal cavity is mandatory to exclude pathological conditions (malignancies, infectious, and other disease processes), which might preclude organ donation. The liver should be evaluated in terms of size and parenchymal quality. A perfect donor liver has sharp edges, a smooth surface with appearance of scratching marks, and a brownish surface color while suboptimal organs might present with round edges, some degree of parenchymal yellowness, absence of scratching marks, and a too soft (steatosis) or increased parenchymal firmness (fibrosis). Based on the initial assessment, the procurement surgeon will decide to proceed or to abort the procurement procedure.
retractor with penetrating clamps is used for appropriate abdominal exposure. If the thoracic cavity is opened, a sternal retractor is placed, which improves the exposure especially of both upper abdominal quadrants. Before the start of surgical maneuvers, a thorough exploration of the liver and the abdominal cavity is mandatory to exclude pathological conditions (malignancies, infectious, and other disease processes), which might preclude organ donation. The liver should be evaluated in terms of size and parenchymal quality. A perfect donor liver has sharp edges, a smooth surface with appearance of scratching marks, and a brownish surface color while suboptimal organs might present with round edges, some degree of parenchymal yellowness, absence of scratching marks, and a too soft (steatosis) or increased parenchymal firmness (fibrosis). Based on the initial assessment, the procurement surgeon will decide to proceed or to abort the procurement procedure.
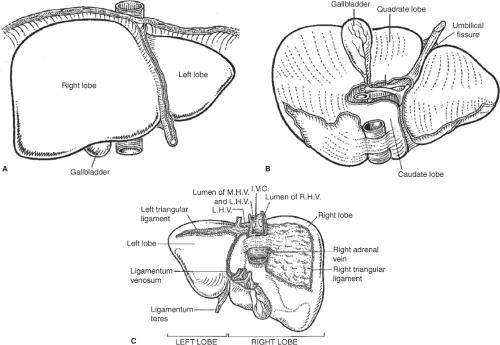
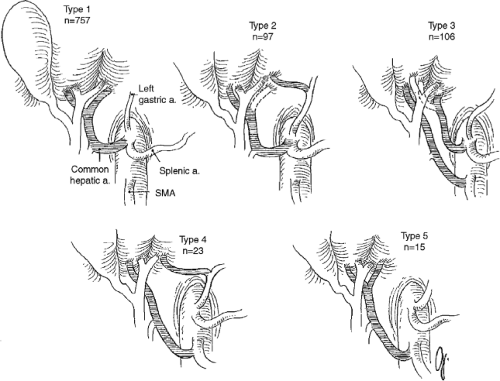
If the assessment of the donor organ is acceptable and there is no thoracic or cardiac organ retrieval planned, the chest is opened through a median sternotomy using a pneumatic saw or, if not available, the Lebsche knife. A self-retaining sternal retractor is used for adequate thoracic exposure. The pericardium is incised through the midline at the diaphragm and cranially extended to the right atrium. The abdominal dissection begins with dividing of the round ligament (ligamentum teres hepatic) between two heavy silk ties. Using electrocautery, the falciform ligament is taken down to the hepatic vein–suprahepatic vena cava confluence and the left coronary and triangular ligaments are divided (Fig. 1). The left lateral segments of the liver (segments 2 and 3) are reflected toward the donor’s right side to access the gastrohepatic ligament. It is of paramount importance to inspect the gastrohepatic ligament for a replaced or accessory left hepatic artery (Fig. 2), which can occur in up to 10% of deceased donors. In the vast majority, a replaced or accessory left hepatic artery originates from the left gastric hepatic artery (type 2) and runs within the gastrohepatic ligament to the umbilical fissure. This artery must be preserved and should be neither injured nor divided. In the presence of a replaced or accessory left artery, the ligament is divided above and below the artery.
The retroperitoneal dissection is initiated with the medial reflection of the ascending colon. The ascending colon and the second (descending) part of the duodenum are mobilized and reflected to the left. Afterward, the remaining small intestine is mobilized cephalad until the left renal vein is identified. Dissections above this level might be harmful due to the potential risk to injure the superior mesenteric artery (SMA), left renal artery, and pancreas. The small intestine is cephalad reflected and the inferior mesenteric vein is isolated at the ligament of Treitz. The vein is distally ligated and then cannulated with a 12 French infusion tubing line for the precool perfusion. We use an isotonic saline solution with 5% dextrose for serum levels less than 160 mEq/gL and 5% dextrose in free water for sodium levels greater than 160 mEq/gL. The volume of the chilled solution, usually not more than 1,000 mL, is slowly infused during the preparation.
The distal abdominal aorta is exposed above the level of the bifurcation in preparation for cannulation. The peritoneum and the periaortal tissue are widely divided at the bifurcation and carried above the origin
of the inferior mesenteric artery. It is recommended that the plane of transection above the aorta should be slightly lateral toward the right to avoid cross-sectional injury to the inferior mesenteric artery. The aorta is encircled with two umbilical tapes: the distal tape is placed at the level of the bifurcation and held by a Kelly clamp while the proximal tape is snared with a Rommel tourniquet and positioned just below the takeoff of the inferior mesenteric artery. In some cases, the inferior mesenteric artery might be divided for better exposure.
of the inferior mesenteric artery. It is recommended that the plane of transection above the aorta should be slightly lateral toward the right to avoid cross-sectional injury to the inferior mesenteric artery. The aorta is encircled with two umbilical tapes: the distal tape is placed at the level of the bifurcation and held by a Kelly clamp while the proximal tape is snared with a Rommel tourniquet and positioned just below the takeoff of the inferior mesenteric artery. In some cases, the inferior mesenteric artery might be divided for better exposure.
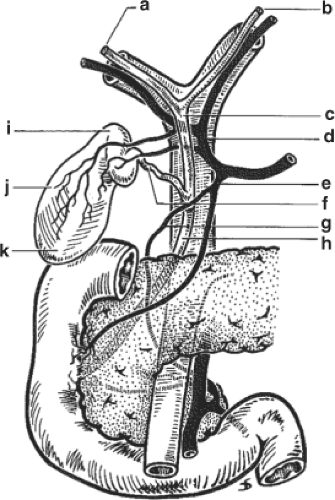
The small intestine and ascending colon are reflected into the abdominal cavity and the porta hepatis is exposed. It is advisable to palpate the posterior hilum to identify a potential replaced right hepatic artery. The common bile duct above the duodenum (Fig. 3) is identified and carefully encircled with 2-0 silk. It is critical that the dissection does not extend into the hilum. The common bile duct is tied with 2-0 silk distally at the duodenal border and is half-way incised just above the tie. The gallbladder fundus is incised and flushed with saline solution until the effluent from the transected bile duct is clear.
The final maneuver before cannulation starts with the exposure of the supraceliac aorta. After the left lobe is elevated and retracted, a longitudinal incision of the right diaphragmatic crus is performed. This maneuver provides adequate access to the supraceliac aorta and might be more difficult in the presence of a replaced left hepatic artery. The preaortic fascia layer is sharply divided laterally with scissors and the completely exposed aortic segment is encircled with an umbilical tape using a blunt-tipped large right-angle clamp. The tape easily identifies the position of accurate aortic cross-clamping.
The patient is then systemically heparinized with 30,000 IU of heparin allowing at least 3 minutes of circulation. After the abdominal aorta is ligated close to its bifurcation with the distal umbilical tape, an aortotomy is made and a 22 French reinforced cardiac catheter is inserted. The catheter is held in place with the Rommel tourniquet and a double ligation that includes catheter and tourniquet. In agreement with all recovering teams, the cross-clamp and the start of the perfusion are initiated. The cross-clamping is performed with a straight vascular clamp at the supraceliac position with the encircled umbilical tape. Immediately after this maneuver, the suprahepatic vena cava is transected at the caval–atrial junction and the dual perfusion with ice-cold preservation solution via abdominal aorta and inferior mesenteric vein is started. Slashed ice is immediately placed around the liver (including subdiaphragmatic space, hilum, and anterior and posterior to the liver), around both kidneys, and, in case of pancreas retrieval, into the lesser sac. We exclusively use University
of Wisconsin (UW) solution at 4°C (39.2°F) as preservation solution infusing 2,000 to 3,000 mL via the aortic cannula and 1,000 mL via the inferior mesenteric vein.
of Wisconsin (UW) solution at 4°C (39.2°F) as preservation solution infusing 2,000 to 3,000 mL via the aortic cannula and 1,000 mL via the inferior mesenteric vein.
After completion of cold perfusion and retrieval of cardiothoracic organs, the pericardium and the left and right diaphragm around the liver are divided. The cold dissection in the hilum starts with the arterial axis. Both the gastroduodenal and splenic artery are divided and the celiac trunk is followed toward the aorta. The splenic vein and the superior mesenteric vein are transected and the portal vein is dissected cephalad. In case of pancreas retrieval, the portal vein is divided to provide sufficient length for both the liver and the pancreas and to avoid pancreatic injury. The transection of the common bile duct is completed at the previous incision. If a replaced left or right hepatic artery is identified, each structure is followed either to the left gastric artery or the SMA (Fig. 2). The hilum dissection is completed after transecting the aorta with an aortal patch including the orifice of the mesenteric artery and celiac artery. The inferior vena cava is divided proximal to the renal veins and the liver is excised and packed in UW solution for transport.
Back-Table Preparation of the Donor Liver
Before the implantation into the recipient, the donor organ has to be prepared, which usually occurs during the recipient hepatectomy. The entire back-table preparation is performed in preservation solution at 4ºC (39.2ºF). The remnant diaphragm is removed and the suprahepatic vena cava is isolated. Phrenic vein orifices of the suprahepatic vena cava are identified and ligated or sutured. Then, the inferior vena cava is separated from adjacent tissue. The inferior vena cava is carefully inspected and the right adrenal vein and other small branches are ligated. The portal vein is isolated to the level of the bifurcation and cannulated for later flush with ice-cold lactated Ringer’s with albumin solution. Finally, the entire arterial axis is skeletonized. Therefore, it is of paramount importance to identify any anatomic variations (Fig. 2) that would require reconstruction (types 2, 3, and 4). Dissection above the level of the gastroduodenal artery should be avoided due to potential injury to the proper hepatic artery.
Recipient Hepatectomy
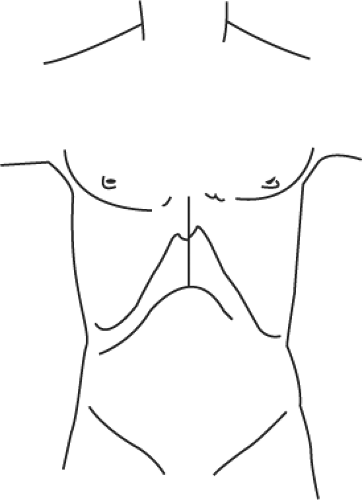
Appropriate access to the upper abdominal cavity is achieved through a bilateral subcostal incision with midline extension (Fig. 4). Except for the skin incision, the subcutaneous tissue, muscle, and fascia layers are divided with electrocautery. The right-sided incision extends more laterally than the left-sided incision. The midline incision is carried out to the xiphoid, requiring in some cases the resection of the xiphoid process. If the recipient has massive ascites, the subcostal incision should be placed 2 to 3 cm lower as compared with in recipients without ascites. The access is completed by dividing and ligating the round ligament. The falciform ligament is divided with electrocautery toward the suprahepatic vena cava. Afterward, a mechanical retractor system is placed with blades under both costal margins. The residual falciform ligament is taken down to the hepatic vein–suprahepatic vena cava confluence and the left coronary and triangular ligaments (Fig. 1) are divided. The left lateral segment of the liver (segments 2 and 3) are reflected toward the recipient’s right side and the gastrohepatic ligament is divided.
The common hepatic artery is dissected and clamped with a bulldog clamp. This step should avoid the arterial dissection after the left and right hepatic arteries are ligated. The hilar dissection begins with incising the peritoneum at the superior border of the hilum in order to identify the left and right and sometimes the middle hepatic arteries. The hepatic arteries are dissected, ligated, and divided. The cystic duct and cystic artery (Fig. 3) are divided, which facilitates the circumferential dissection of the common hepatic duct. The isolated duct is then divided high in the hilum to ensure sufficient length of the recipient’s duct for the later biliary anastomosis. In many cases, the dissection and division of the right hepatic artery is only possible at this point since the right hepatic artery crosses posteriorly to the common hepatic duct (Fig. 3). We then separate the arterial axis (proper hepatic artery) from the common bile duct. We routinely divide the gastroduodenal artery and follow the dissection toward the common hepatic artery until the position of the previously placed bulldog clamp. The portal vein is now skeletonized just until above the pancreatic border. During the distal dissection, the surgeon will find anterior and lateral portal vein branches that have to be ligated and divided. The portal vein dissection is completed by removing the posterior plane of lymphatic and connective tissue.
The right lobe and the inferior vena cava are now mobilized. The right lobe is carefully elevated and the right coronary and triangular ligaments (Fig. 1) are taken down with electrocautery. The dissection begins at the lateral inferior aspect and divides the ligament carefully all the way into the vena cava. After the peritoneum overlaying the anterior part of the infrahepatic vena cava is divided, the line of peritoneal dissection follows the plane between left-sided vena cava and the peritoneal sheet all the way up to the suprahepatic vena cava. This maneuver often provides the window underneath the hepatic vena cava to the right side. The dissection is completed now on the right side. The right lobe is elevated and the right adrenal vein is ligated and divided. Finally, the peritoneum is divided longitudinally along the vena cava. This results in the complete separation of the posterior aspect of the retrohepatic vena cava from the retroperitoneum.
The native liver is now ready for removal if no venovenous bypass (VVB) is applied. Vascular clamps are placed on the distal portal vein, infrahepatic and suprahepatic vena cava (Fig. 5). It is important that all clamps be oriented horizontally with the liver in the normal anatomic position to avoid rotation of the vessels. We use a large Satinsky vascular clamp for the suprahepatic vena cava, which should be placed on the very edge of the diaphragmatic reflection to avoid injuries to the phrenic nerve. The suprahepatic vena cava is first divided far into the hepatic veins. Next, the lower cava is divided as proximal as possible and finally the portal vein. The native liver is now removed (Fig. 6). Hemostasis of the bare area is achieved by retroperitonealization (Fig. 6, inlay). We use running sutures with 2-0 Prolene staring laterally and running centrally followed by a second running suture in the midline. For recipients with HCC, no residual liver tissue of the native liver should remain at the suprahepatic vena cava.
Venovenous Bypass
The VVB drains the infrahepatic caval and splanchnic blood into the superior vena cava (Fig. 7). Although not yet proven in randomized controlled trials, the VVB offers several advantages. The main advantage is related to the maintenance of venous return and splanchnic venous drainage, which results in improved hemodynamic stability during the anhepatic phase and reduction in mesenteric edema. The introduction of the VVB resulted initially in a decline of early morbidity and mortality; however, VVB is not routinely used and is likely less necessary for cava-sparing techniques (piggyback). In general, VVB is indicated in patients with hemodynamic instability after clamping, with fulminate hepatic failure to reduce volume overload, and with non-dialysis-dependent hepatorenal syndrome.
VVB of the subclavian and femoral vein can be performed as either a percutaneous or open approach (Fig. 7). In both techniques, the cannulation of the portal veins is identical and performed by the surgeon. In the percutaneous technique, the femoral vein and the subclavian vein or alternatively the right internal jugular vein (IJV) are punctured and cannulated via the Seldinger technique. We use wire-inforced cannulas, 15 French for the femoral vein and 12 French for the subclavian vein or right IJV. In the open technique, we cut down on the left axillary vein and left saphenofemoral junction using 20 and 18 French cannulas, respectively. The cannulation of the saphenous vein should always be approached. In absence of the saphenous vein, the cannulation of the femoral vein is possible but requires vascular reconstruction after removal of the cannula. We usually start the cannulation of the portal vein with a ligation high up in the hilum. After placing an umbilical tape around the vein and a vascular clamp on the distal part of the portal vein, the proximal portal vein is incised and 22 French wire-reinforced cannula is introduced and advanced after removal of the distal vascular clamp. The cannula is secured with tourniquet and ligations.
Implantation of Cadaver Whole Allograft
Suprahepatic Vena Cava Anastomosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree