Fig. 1
Light sheet-based fluorescence microscopy (LSFM). (a) Setup of a single plane illumination microscope (aka SPIM) [5]). (b) Principles of LSFM imaging. Left : a single plane in the specimen is illuminated by a light sheet. Only the plane that is observed is also illuminated, resulting in lower photobleaching and lower phototoxicity. Center : by moving the specimen through the stationary light sheet, a three-dimensional stack of images is recorded. Right : by rotating the specimen multiple-view image stacks are obtained. Combining multiple different views of the specimen increases the resolution along the z-axis. (c) Close-up of a specimen chamber, showing both the illumination and detection objective lenses oriented at a 90° angle with respect to each other
LSFM is also extremely well suited to record the behavior of live mammalian cells for long periods of time [10] and is the tool of choice for imaging three-dimensional cell cultures, such as cellular spheroids. The latter become more and more popular in basic research [11], for drug screening [11–15], and for personalized medicine [16]. Most immortalized and tumor cell lines, primary cells, as well as stem cells can form spheroids by spontaneous aggregation on nonadhesive substrate [17]. The spheroid formation consists of aggregation, delay, and compaction phases. The entire process takes 3–7 days [18]. Previous work has shown that LSFM is particularly well suited to image tumor cell spheroids in three dimensions at high resolution [19] and with live cells [20].
We show how to perform long-term live imaging by combining the LSFM with a perfusion chamber. Agarose beakers are employed to allow the cell aggregation in a confined environment. As an example, we record the complete formation of a spheroid over a time period of 6 days. We use the recently developed hepatic human cell line HepaRG, which is well suited for drug and toxicity screenings [21].
2 Materials
2.1 Chemicals and Reagents
1.
Cell growth medium: Add 50 ml FBS (100 % fetal bovine serum), 5 ml penicillin/streptomycin, and 5 ml 200 mM l-glutamine to 445 ml DMEM (1× Dulbecco’s modified eagle medium phenol-free). Mix 500 ml of medium. Store at 4 °C.
2.
Cell dissociation: StemPro Accutase Cell Dissociation Reagent (Life technologies, #A1110501).
3.
Agarose aliquots: Prepare a 1 % solution of high-melting point agarose (Sigma, A9539) in PBS. Aliquot the agarose solution in 2 ml tubes. Store at 4 °C.
4.
Transduction reagent: BacMam viral particles containing a histone 2B-GFP expression cassette (Invitrogen C10594).
2.2 Cell Culture Equipment
2.
Hemocytometer (e.g., Neubauer hemocytometer).
2.3 Cell Line
HepaRG, terminally differentiated hepatic cells derived from a human hepatic progenitor cell line that retains many characteristics of primary human hepatocytes (e.g., from Life Technologies, HPRGC10).
2.4 Templates for the “Agarose Beakers”
Custom templates, see further in the text.
2.5 Further Equipment
2.
1 ml syringes.
3.
0.55 × 25 mm (24 G × 1″) hypodermic needles.
4.
Custom LSFM mounting system for the agarose beaker (details further in the text).
2.6 Imaging
1.
Various suitable LSFM implementations are described in details in [5, 22, 23] (SPIM), (6) (DSLM), and [8] (monolithic DSLM, mDSLM). A commercial LSFM is available from Zeiss (Lightsheet Z.1, http://microscopy.zeiss.com/microscopy/en_de/products/imaging-systems/lightsheet-z-1.html#Introduction).
2.
Long working water-dipping distance objective lenses (e.g., Carl Zeiss W N-Achroplan 10×/0.3 NA).
2.7 Temperature and Gas Control System for Time-Lapse Live Imaging
1.
A compact cell incubator (e.g., Galaxy® 14S CO2 incubator, New Brunswick http://eshop.eppendorfna.com/products/New_Brunswick_Galaxy_14S_CO2_Incubator).
3.
Microprocessor-controlled table-top temperature controller (e.g., WRT2000X, from Winkler http://en.winkler.eu).
4.
A 4 meter-long gas-permeable silicon tubing (e.g., from Reichelt Chemietechnik, Germany, 1 mm internal diameter, 2 mm external diameter, http://www.rct-online.de).
5.
Gas-impermeable rubber tubing (e.g., from Reichelt Chemietechnik, Germany—EPDM/PP pharmaceutical tubing, 1.6 mm internal diameter, 4.8 mm external diameter, http://www.rct-online.de).
6.
Peristaltic pump (e.g., REGLO digital from Ismatec, Germany, http://www.ismatec.de/de_d/pumpen/s_reglo/reglo_digital.htm).
7.
Two-stop tubing for the peristaltic pump (e.g., 1.30 mm two-stop PharMed BPT tubing, Ismatec SC0328).
8.
Autoclavable Luer-Lok connectors, mini tubing olive connectors (inner Ø 2 and 1.5 mm), and quick-disconnect coupling/nipple systems with valve (e.g., THOMAFLUID-POM with 1.6 mm nozzle). All this part can be purchased, e.g., from Reich elt Chemietechnik, Germany, http://www.rct-online.de).
2.8 Software for Image Acquisition and Image Processing
Image processing software (e.g., Fiji, a variant of ImageJ, http://fiji.sc).
3 Methods
3.1 Thawing of the HepaRG Cell and Transduction with the Histone 2B-GFP Nuclear Marker
1.
Rapidly thaw the frozen HepaRG cells (within 5 min) in a 37 °C water bath. Dilute the thawed cells in a 5 ml pre-warmed growth medium. Centrifuge cells for 4 min at 300 × g. Resuspend the pellet in 5 ml pre-warmed cell growth medium. Plate cells at high density in a 25 cm2 cell culture flasks to speed up recovery. Change medium after 24 h. Propagate cells at 90–100 % confluence. Detach cells with 500 ml StemPro Accutase Cell Dissociation Reagent, and resuspend cells in 4.5 ml cell growth medium.
2.
Determine the concentration of cells in the medium with a hemocytometer (e.g., Neubauer hemocytometer).
3.
Expand HepaRG cells as monolayer on a 75 cm2 tissue culture flask, until they reach a 70 % confluence.
4.
Add the BacMam transduction reagent with a histone 2B-GFP expression cassette to the plated cell, by pipetting the viral particles directly in the media at the density recommended by the company data sheet. Baculoviral vectors for fluorescent labeling of the cell nuclei have a relatively low cellular toxicity [24]. After 24 h of incubation with the BacMam particles, cells are detached by employing StemPro Accutase and the cell suspension is diluted to 2 × 103 cells in 25 μl (the volume of the measuring chamber mounted in the LSFM). The transduction efficiency approaches 90 % (Fig. 2).
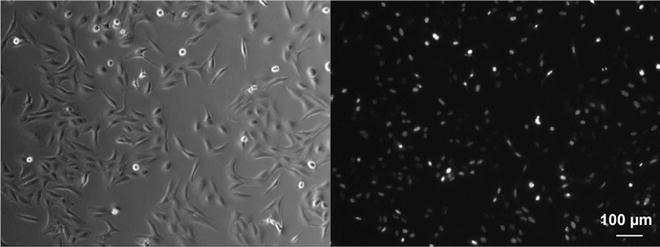

Fig. 2
HepaRG cells transduced with nuclear marker H2B-GFP. Left : transmitted light image. Right: fluorescence image. Objective lens: CZ Achroplan 10×/0.3. Ex/Em: 488/520 nm. Microscope Zeiss Axiovert 40 CFL
5.
Perform a control experiment by forming HepaRG spheroids in a suitable U-well plate (e.g., the HydroCell Surface™ 96-well plate). The original cell suspension is diluted in growth medium, and 100 μl is transferred in each well. An 8-channel pipette can be employed to reduce the pipetting time. Incubate the multiwell plate under standard cell culture conditions. Spheroid formation is completed after 2–6 days (Fig. 3).
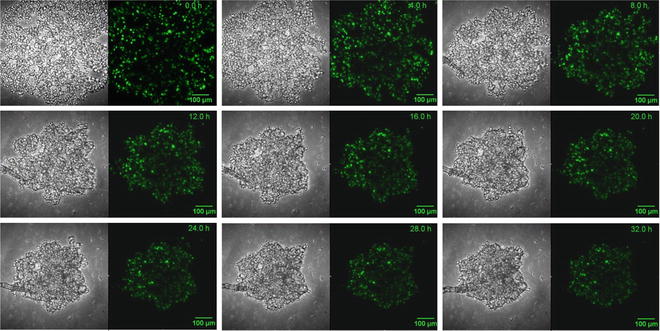

Fig. 3
Aggregation of a HepaRG spheroid for 32 h. 2,000 cells/well expressing H2B-GFP were seeded into a 96-well plate coated with an agarose layer. The cells do not adhere to the agarose layer and form compact spheroids within days (aka liquid overlay method). Transmitted light and fluorescence images are shown. Objective lens: Nikon CFI Plan Fluor 10×/0.30, WD 16 mm. Ex/Em: 488 nm/515 nm. Time-lapse interval: 30 min. Microscope: Nikon Eclipse confocal fluorescence microscope
3.2 Agarose Beaker Molding
1.
A caster can be made from an aluminum tube and an aluminum plunger (Fig. 4a, b). Employ aluminum or stainless steel for both the tube and the plunger to support the rapid cooling of the agarose gel. Use a conically shaped plunger tip to simplify the aggregation of the cells in the center of the beaker (Fig. 4a, b, see Note 1 ).
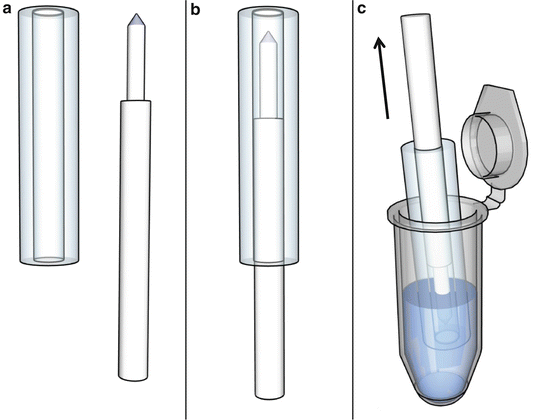

Fig. 4
Schematic representation of the template and the production of agarose beakers. (a) The template consists of a stainless steel barrel and a plunger. (b) The assembled template. (c) The liquid 1 % high-melting agarose is sucked into the template by pulling the plunger
2.
Autoclave the caster.
3.
Prepare a 1 % solution of high-melting point agarose in PBS and aliquot it in 1.5 ml tubes.
4.
Place few aliquots of the 1 % agarose solution in a heating block at 95 °C for 15 min in order to completely melt the agarose.
5.
Working under the laminar flow sterile hood, immerse the caster in one 1.5 ml tube with the liquid agarose solution. Suck the agarose into the template by pulling up the plunger (Fig. 4c). Place the caster at 4 °C for 5 min to allow a fast hardening of the agarose gel.
6.
Get Clinical Tree app for offline access

After agarose hardening, push the plunger out of the template. Separate the agarose beaker from the plunger by completely immersing it in PBS and by gently pushing the beaker out with a sharp angled tip precision forceps (Fig. 5).
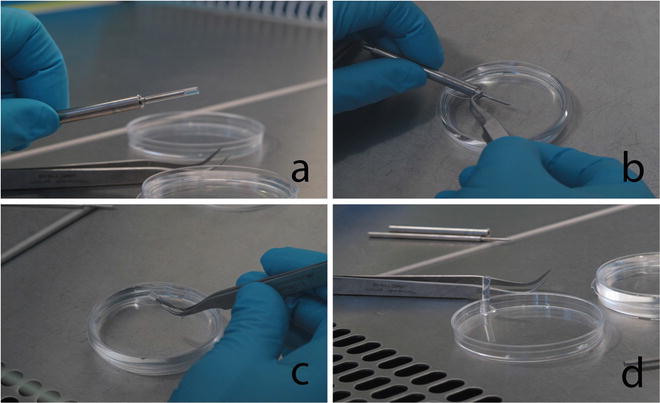

Fig. 5




The photographs illustrate the extraction procedure of the agarose beaker from the stainless-steel template. (a) The agarose beaker is still on the template. (b) The agarose beaker is immersed into sterile PBS and gently pushed out of the template with a sharp-tip angled forceps. (c) The agarose beaker is carefully manipulated with the forceps, and (d) the beaker is placed vertically in an empty petri dish in order to fill it with the cell suspension
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


