chapter 4 Lipids: fats and oils
Lipids are a diverse collection of small, water-insoluble (hydrophobic) molecules mostly consisting of hydrocarbons but also have some polar bonds associated with oxygen. There are many different types of lipids but this chapter will focus on the most biologically relevant types: fatty acids, triacylglycerols, glycerophospholipids, sphingolipids and steroids.
Fatty acids
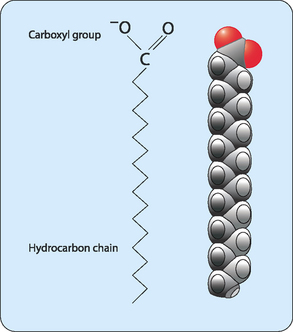
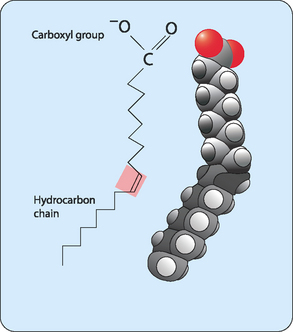
Fatty acids are the simplest lipids. In humans fatty acids normally consist of a carbon skeleton of 16 or 18 carbons, with a carboxylic acid group—which gives the molecules their name fatty acids—at one end (Fig 4-1). Fatty acids with fewer than 14 or more than 20 carbons are rare but most have an even number of carbon atoms. The majority of these molecules consist of nonpolar C–H bonds, making the majority of the molecule hydrophobic. However, the carboxylic acid group is hydrophilic, making these molecules amphipathic. This means that part of the molecule ‘loves’ water whereas another part ‘hates’ water.
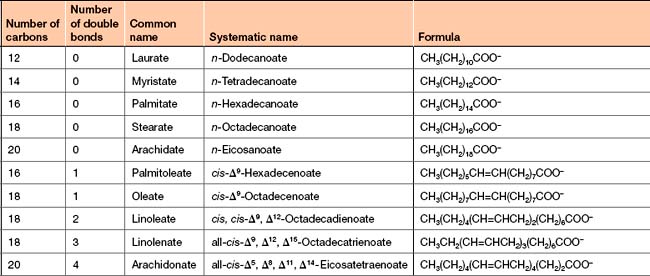
The naming of fatty acids can be confusing as each molecule has a common name, which usually relates to what it was originally isolated from, and a systematic chemical name. In biochemistry the common names of these molecules are often used. This means an awareness of each of the names for these molecules is required since the common name does not give structural information. The systematic chemical name for a fatty acid is formed by taking the number of carbons in the molecule and adding the ending ‘anoic’ to define it as an acid. This means that a fatty acid containing 16 carbons is called hexadecanoic acid (hexa = 6; dec = 10). The names of common fatty acids are given in the accompanying table (Table 4-1). The number of carbons in a fatty acid affects its physicochemical properties. As the number increases the melting point also increases but its solubility in water decreases dramatically (Table 4-2).
TABLE 4-2 The effect of the number of carbons in a fatty acid molecule with its physical and chemical properties
| Carbons | MP (°C) | mg/100 mL H2O |
|---|---|---|
| 4 | −8 | – |
| 6 | −4 | 970 |
| 8 | 16 | 75 |
| 10 | 31 | 6 |
| 12 | 44 | 0.55 |
| 14 | 54 | 0.18 |
| 16 | 63 | 0.08 |
| 18 | 70 | 0.04 |
MP, melting point (°C)
mg/100 mL H2O, amount of each fatty acid that can dissolve in 100 mL of water.
Saturated or unsaturated?
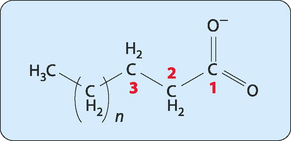
About 50% of the fatty acids in human cells are referred to as unsaturated. This means that these fatty acids contain at least one double bond in their hydrocarbon chain (Fig 4-2). Polyunsaturation is common, with more than one double bond being present, but the first double bond is nearly always found between C9 and C10 (see Fig 4-3 for the numbering of carbons in fatty acids). The double bonds are always in the cis conformation and are never conjugated (e.g. if there is a double bond between C9 and C10 then there will not be one between C8 and C9 or between C10 and C11). The systematic chemical name for an unsaturated fatty acid is formed in a similar manner to that of a saturated one, first, by taking the number of carbons in the molecule but this time adding the ending ‘enoic’ (rather than ‘anoic’) to define it as an unsaturated acid. This means that a fatty acid containing 16 carbons with a single double bond is called hexadecenoic acid. If there are two double bonds the ending becomes ‘dienoic’, for three double bonds ‘trienoic’ and for four double bonds ‘tetraenoic’.
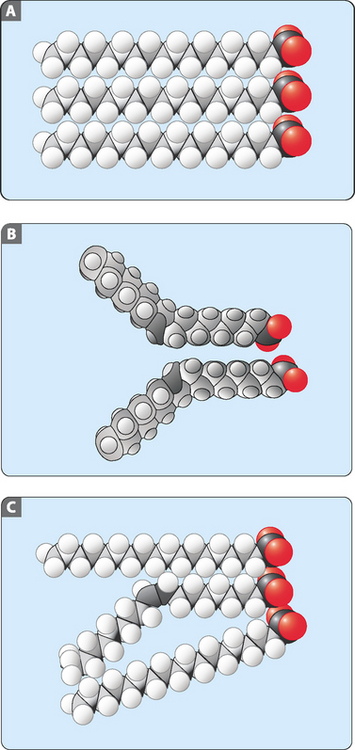
The addition of double bonds has striking effects on the physicochemical properties of these molecules. In particular, the addition of a single double bond to a fatty acid considerably decreases its melting point (Table 4-3). This effect occurs because the double bond alters the packing of the molecules (Fig 4-4). Saturated (rod-like) molecules are able to pack closely together and interact over the majority of their length (Fig 4-4A). This interaction requires a lot of energy (e.g. heat) to break, resulting in a high melting point. Unsaturated (bent) molecules cannot pack so closely and do not interact as greatly as saturated ones (Fig 4-4B). This means that these interactions require less energy (e.g. heat) to break, resulting in a lower melting point. When a mixture of saturated and unsaturated molecules is examined the effect of unsaturated fatty acids on packing is very obvious. These effects on packing, and the resultant melting points, carry over to molecules made from fatty acids, for example, triacylglycerols and glycerophospholipids.
TABLE 4-3 The effect of double bonds on the melting point of fatty acids
| FA (C: Double bonds) | MP |
|---|---|
| 16:0 | 60 |
| 16:1 | 1 |
| 18:0 | 63 |
| 18:1 | 16 |
| 18:2 | 5 |
| 18:3 | −11 |
| 20:0 | 75 |
| 20:4 | −50 |
MP, melting point (°C)
Some fatty acids improve human health
Many studies have shown that diets rich in saturated fat and cholesterol are linked with an increased risk of heart disease whereas diets high in unsaturated fat and low in cholesterol lower the risk of heart disease. In particular, there are several types of unsaturated fatty acids which appear to mediate the protective effect. There are a number of fatty acids, referred to as essential fatty acids, which cannot be synthesised by the body and which are important precursors of many biologically active and clinically relevant molecules. The most important for humans are ω(omega)-3 fatty acids found in oily fish such as salmon, herring, mackerel, sardines and anchovies and ω-6 fatty acids found in vegetable oils, particularly those derived from seeds (Fig 4-5). A dietary deficiency of ω-3 and ω-6 fatty acids can lead to dry, scaly skin, poor wound healing and hair loss. On the other hand, populations in which the dietary intake of these essential fatty acids is higher than average have much lower than average incidences of coronary heart disease, diabetes and rheumatoid arthritis. This has led to the conclusion that increased consumption of ω-3 and, in particular, of ω-6 fatty acids reduces cholesterol levels and decreases the incidence of heart disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree