![]() LEARNING OBJECTIVES
LEARNING OBJECTIVES
After reading this chapter, the pharmacy student, community practice resident, or pharmacist should be able to:
1. Describe lipids and lipoproteins and their associated disorders.
2. Identify the risk factors associated with the development of lipoprotein disorders.
3. Review prescription and over-the-counter (OTC) pharmacotherapy treatment options for lipoprotein disorders.
4. Apply patient case and pharmacist interviews to overcome barriers to implementation of lipid management services within the community pharmacy setting.
5. Analyze the business management aspects needed to create lipid management services in a community pharmacy.
 INTRODUCTION TO LIPID DISORDERS
INTRODUCTION TO LIPID DISORDERS
Description of Lipids and Lipoproteins
Cholesterol, triglycerides, and phospholipids are transported through the blood in particles made up of lipids and proteins (lipoprotein complexes). Low-density lipoprotein (LDL), high-density lipoprotein (HDL), and very low-density lipoprotein (VLDL) are the three main types of lipoprotein complexes present in serum. Intermediate-density lipoprotein (IDL) is included in the LDL measurement in clinical practice but is located between VLDL and LDL. Chylomicrons are lipoproteins that are formed in the intestine and carry triglycerides from dietary fat.
LDL (60–70% of total cholesterol) contains the apolipoprotein (apo), apo B-100, and is the primary target of cholesterol-lowering agents due to its atherogenic properties, while HDL (20–30% of total cholesterol) carries cholesterol from lipid-rich foam cells to the liver and may protect against atherosclerosis. HDL contains apo A-I and apo A-II. VLDL (10–15% of total cholesterol) is rich in triglycerides and contains apo B-100, C-I, C-II, C-III, and E. Chylomicrons not only contain apo C-I, C-II, C-III, and E but contains B-48 in the place of B-100.
Overview of Lipid Disorders
Lipoprotein disorders are classified based on the specific lipoproteins involved resulting from primary or genetic defects (see Table 8–1). There are also many secondary causes of lipoprotein disorders that must be considered when suggesting, initiating, or modifying therapy (see Table 8–2).
Table 8–1. Definition, Classification, Characteristics, and General Treatment of Lipid Disorders
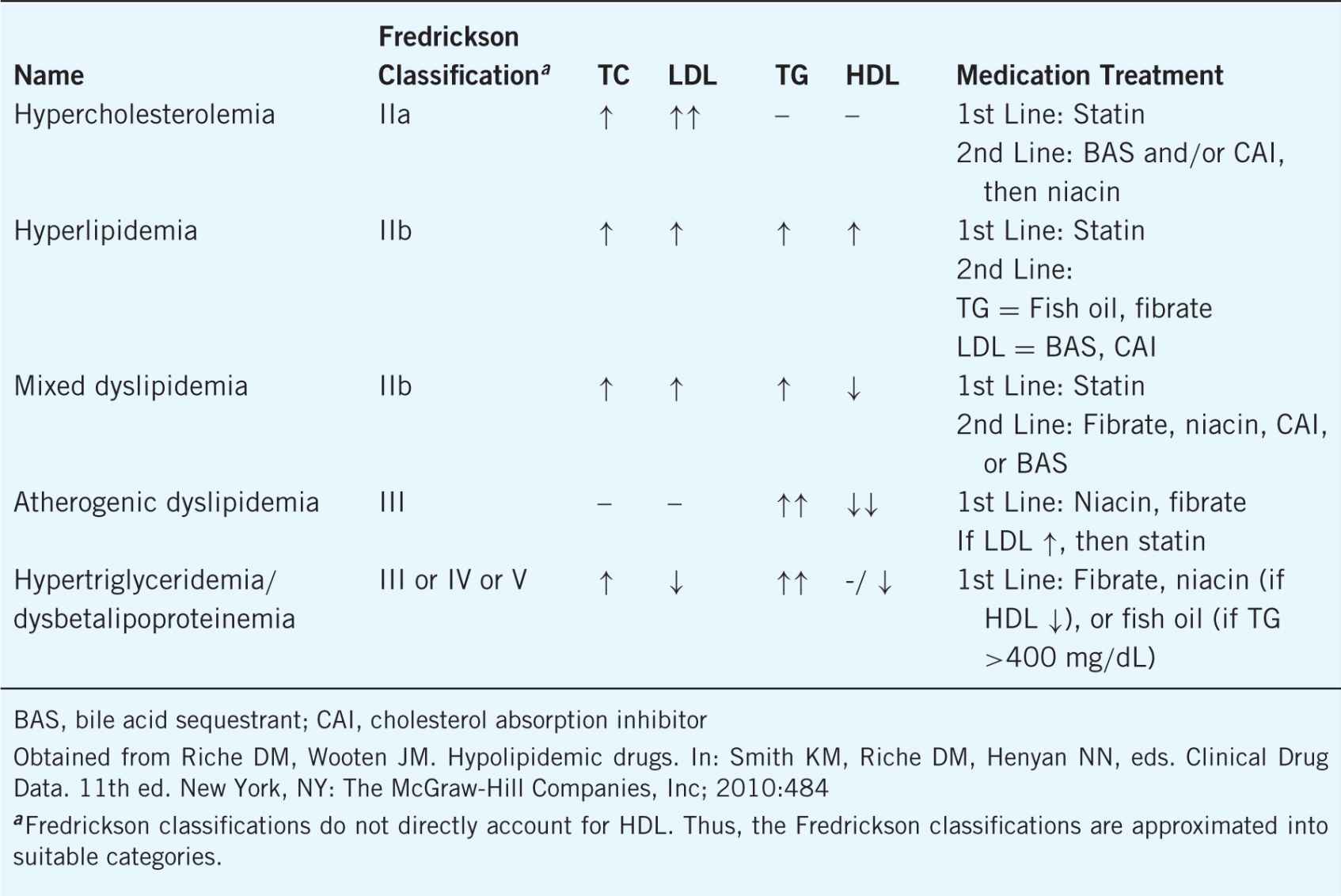
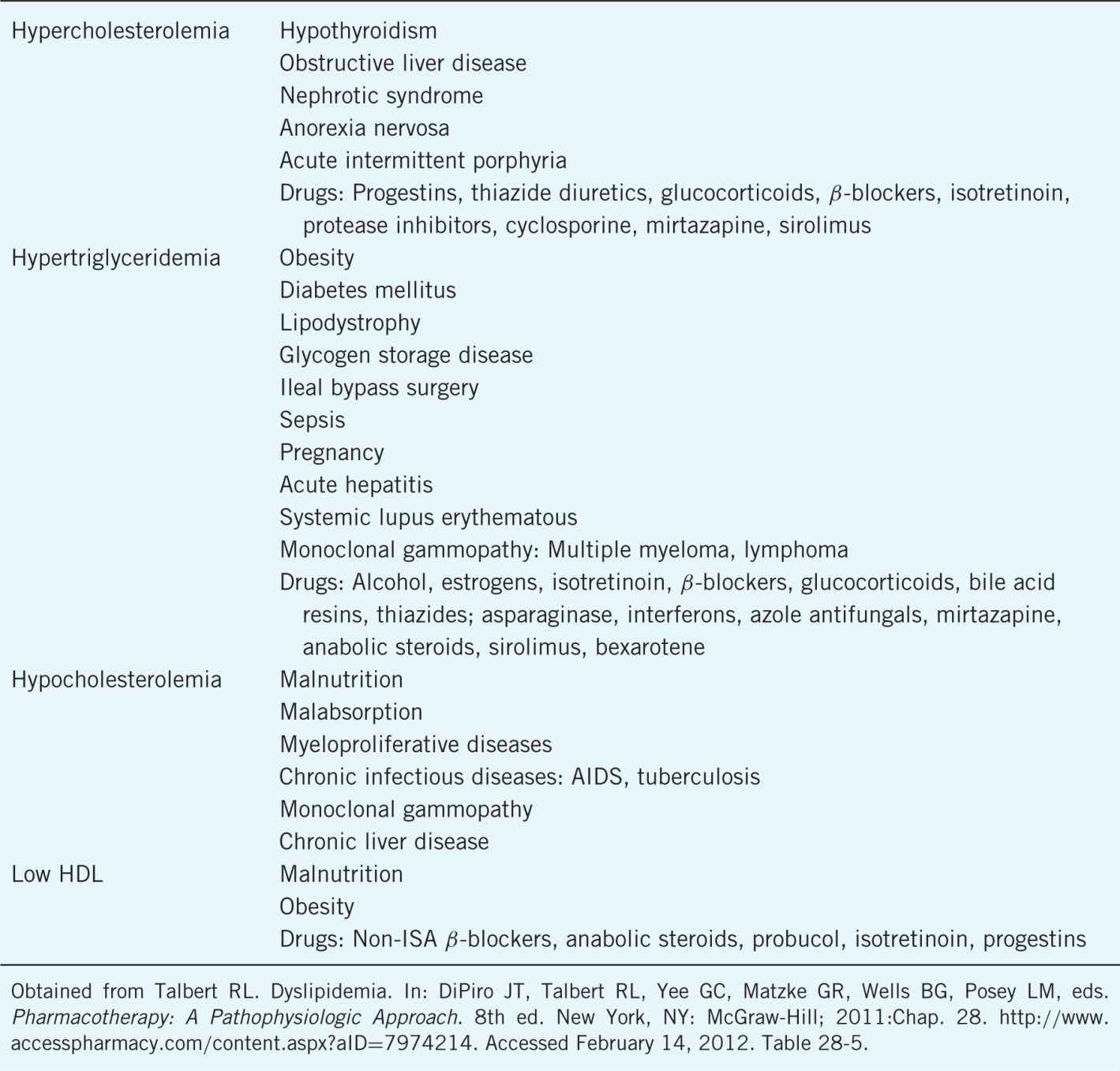
Table 8–2. Secondary Causes of Lipid Disorders
 PATIENT EVALUATION
PATIENT EVALUATION
Classification of Lipoprotein Levels
A lipid panel (after a 12-hour or longer fast) should be drawn every 5 years for all adults 20 years of age or older. The panel should include total cholesterol, LDL, HDL, and triglycerides. Since LDL is a calculated value, only total cholesterol and HDL are accurate if the patient is in a non-fasting state. Triglycerides may be falsely elevated when drawn non-fasting, resulting in the appearance of lowered LDL.
Identification of Risk
During lipid screenings or lipid management, the pharmacist should complete a detailed patient history. This history must assess traditional cardiovascular risk factors (see Table 8–3), as well as an assessment of emerging risk factors. Identification of physical manifestations of lipid disorders (e.g., xanthomas, abdominal pain, or pancreatitis history) should be considered.
Table 8–3. Traditional Cardiovascular Risk Factors
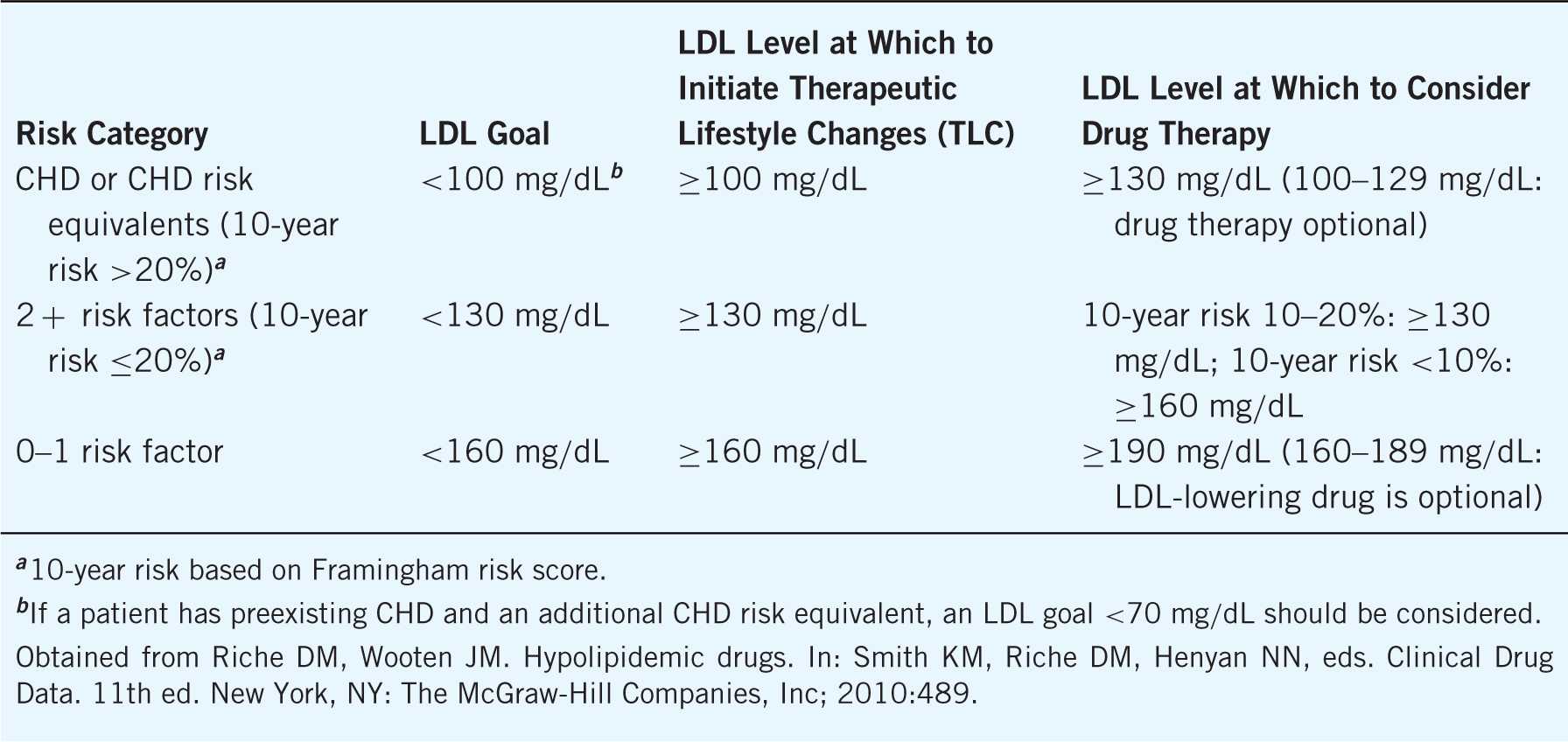
Other than coronary heart disease (CHD) itself, several risk equivalents convey a high chance of CHD development. Based on the ATP III, the most widely accepted risk equivalents include diabetes mellitus (DM), peripheral artery disease (PAD), abdominal aortic aneurysm, cerebral vascular disease (CVD), or a Framingham score >20%. By stratifying patients based on their personal cardiovascular risk factors, pharmacists are able to determine individualized cholesterol goals (see Table 8–4).
Table 8–4. Risk Stratification
Emerging Risk Factors
Many emerging risk factors have been identified over the past several decades to assist in the predication of CHD development. Ideally, emerging risk factors with the highest risk of CHD development could be considered for individualization of cholesterol goals and treatment. Considering the extended duration of time since the last ATP III update, the concept of emerging risk factors relating to lipid disorders has increased in popularity. In fact, the Emerging Risk Factor Collaboration (EFRC) has been established to improve the predictive value of emerging risk factors based on evidence.
The EFRC has clearly identified lipoprotein (a) as an independent predictor ofCHD. C-reactive protein (CRP) has also demonstrated a continuous association with CHD, but CRP is also a general marker of inflammation that could indicate less specific predictive potential. A large, randomized, placebo-controlled trial investigated the potential of lipid-lowering therapy (rosuvastatin 20 mg/day) in patients without preexisting dyslipidemia but with elevated CRP. Results demonstrated significant benefit of lipid-lowering therapy, possibly confirming the role of CRP as an independent CHD risk factor.
Coronary artery calcium (CAC) scores have been evaluated as an adjunct to traditional Framingham risk methods. Higher CAC scores (particularly >400) have been significantly associated with progressive stenosis independent of traditional risk factors. The most promising use of CAC scores may be in the moderate classification (Framingham risk 10–20%) to help determine aggressiveness of medication therapy. Two concomitant disease states have also been identified as potential risk factors for CHD: obstructive sleep apnea (OSA) and human immunodeficiency virus (HIV).
Emerging risk equivalents are also under investigation. Chronic kidney disease (CKD), defined as a glomerular filtration rate <60 mL/min/1.73 m2, has been identified by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) as a CHD risk equivalent. This stance is commonly implemented in clinical practice and is further substantiated by the Study of Heart and Renal Protection. The increased awareness of metabolic syndrome has impacted the field oflipid management over the past decade. Metabolic syndrome is a constellation ofat least three metabolic characteristics including low HDL, elevated TG, glucose intolerance, hypertension, and increased waist circumference. The presence of metabolic syndrome confers similar CHD risk to its individual components and provides little additional predictive potential.
Other emerging risk factors mentioned in the ATP III have an unclear impact on CHD development or prevention. Particularly, carotid intimal medial thickening (CIMT), apolipoproteins, cholesterol ratios, and thrombogenic factors have not been consistently targeted as mechanisms of CHD prevention and treatment.
Therapeutic Lifestyle Changes
Vital to the management oflipid disorders is therapeutic lifestyle changes (TLC), a synergistic element with pharmacological therapy. The key components related to TLC are dietary intervention and increased physical activity. Similar to goal-based strategies for cholesterol, TLC goals should be set at an initial visit and evaluated at each subsequent visit. Exercise goals should consist of 30–60 minutes of moderate intensity continuous aerobic physical activity (based on tolerance) including intermittent resistance training on 5–7 days weekly. Dietary interventions are summarized in Table 8–5. Body weight should be reduced up to 10% in 12 months and continued until a body mass index (BMI) below 25 kg/m2 is reached. Waist circumference goals (<40 inches in men; <35 inches in women) can also be considered. Other beneficial lifestyle modifications to cardiovascular risk should be emphasized including smoking cessation, salt-restricted diets for patients with hypertension, and supplemental soluble fiber. Strict compliance with TLC goals can lead to LDL reductions up to 30%. There are several Internet-based resources related to dietary intervention (see http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/Nutrition-Center_UCM_001188_SubHomePage.jsp; http://www.nhlbi.nih.gov/health/index.htm).
Table 8–5. Therapeutic Dietary Recommendations

 MEDICATION MANAGEMENT
MEDICATION MANAGEMENT
LDL-Lowering Agents
HMG CoA Reductase Inhibitors (Statins)
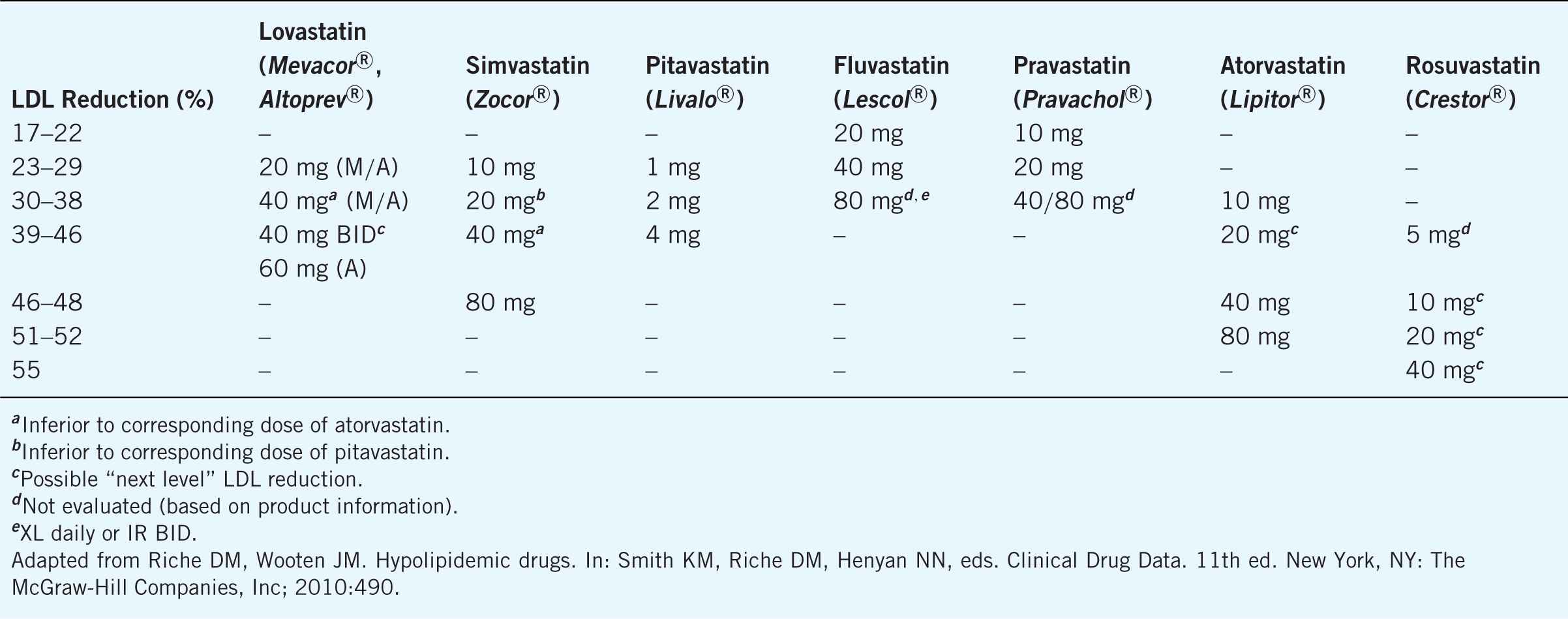
Hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitors (atorvastatin, fluvastatin, lovastatin, pravas-tatin, pitavastatin, rosuvastatin, simvastatin) are the most effective medication class in lowering LDL levels with the potential for decreasing LDL 18–55%, triglycerides 7–30%, and increasing HDL 5–15% (see Table 8–6). Statins have been shown in clinical trials to have favorable outcomes in CHD and CVD due to their beneficial effects on the atherosclerotic process. Statins are generally indicated for the treatment of type IIa and IIb hyperlipoproteinemias.
Table 8–6. Approximate Dose Equivalence of Statins Based on LDL
Statins work by inhibiting HMG-CoA reductase, which is the rate-limiting step in the synthesis of cholesterol. Statins are usually administered in the evening and have a high first-pass clearance by the liver and a short half-life, except atorvastatin and rosuvastatin that can be administered any time of the day due to longer half-lives.
Statins are generally well tolerated. Elevated hepatic transaminases rarely occur, but liver function tests must be monitored. Statins are contraindicated in active or chronic liver disease. Statin-induced myopathy can develop, and changes in creatinine phosphokinase (CPK) should be assessed. Myopathy is more common when statins are used in combination with medications metabolized by cytochrome P450–3A4, including cy-closporine, fibrates, macrolide antibiotics, certain antifungals, and nicotinic acid. Patients should report muscle pain or weakness and the presence of dark red/brown urine. Myopathy can be characterized by myalgia (lacking CPK elevation), myositis (CPK 2–3 times the upper limit of normal), and rhabdomyolysis (CPK 10 times the upper limit of normal). If rhabdomyolysis is suspected, the statin should be promptly discontinued and the patient should seek immediate care.
Ezetimibe
Ezetimibe selectively inhibits the sterol transporter at the brush border of the small intestine, which results in the reduced absorption of dietary and biliary cholesterol. Synergistic lowering of LDL occurs when ezetimibe is combined with statin therapy and ezetimibe is primarily utilized in this manner (See Table 8–7). Because less cholesterol is delivered to the liver, LDL receptors are upregulated and blood cholesterol is reduced.
Table 8–7. Cholesterol Effects of Medications for Lipid Disorders When Combined with a Statin
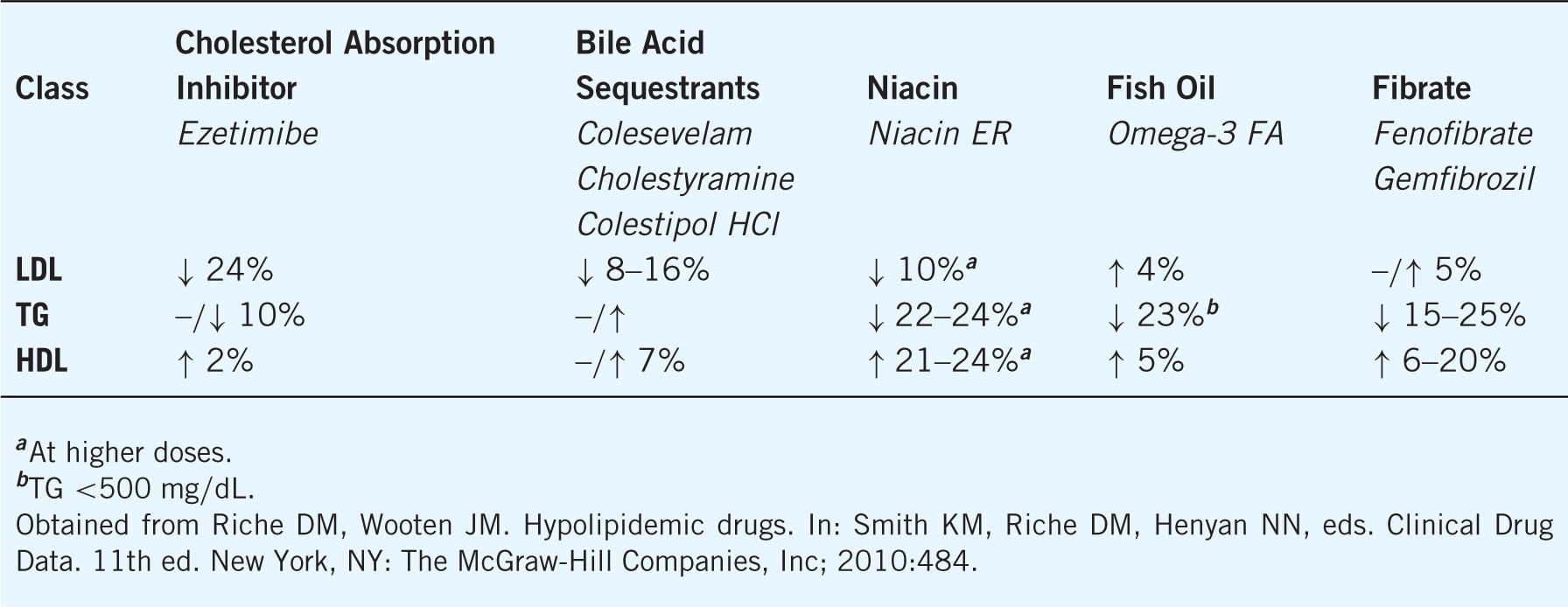
Ezetimibe is relatively well tolerated with myalgias, diarrhea, and upper respiratory tract infections being the most common side effects. Hepatitis, elevated transaminases, arthralgia, and rhabdomyolysis can occur.
Bile Acid Sequestrants
Bile acid sequestrants (cholestyramine, colesevelam, colestipol) decrease LDL 15–30%, increase HDL 3–5%, and have either no effect on triglycerides or result in a slight increase. Their LDL-lowering effect is additive when combined with other agents, and beneficial outcomes have been demonstrated in clinical trials. Sequestrants are indicated for the treatment of primary hypercholesterolemia (types IIa and IIb) in patients whose triglycerides are below 400 mg/dL.
Sequestrants work by binding bile acids in the intestine via anion exchange and reduce their enterohepatic recirculation. In turn, cholesterol is oxidated to form new bile acids. LDL-receptor expression is enhanced in response to less cholesterol being produced. LDL is therefore decreased in the serum. Hepatic production of VLDL may increase in some people and lead to an increase in triglycerides.
Bile acid sequestrants pass through the intestine with systemic absorption or toxicity. Sequestrants can interfere with the absorption of other medications, which should be taken at least 4 hours prior to the sequestrant. Gastrointestinal symptoms, including constipation, bloating, abdominal pain, bloating, fullness, nausea, and flatulence, are common.
Nicotinic Acid (niacin)
Nicotinic acid lowers LDL 5–25%, triglycerides 2050%, and increases HDL 20–50%. Niacin, in combination with other lipid-lowering therapy, has been shown to slow atherosclerotic progression, reduce the risk of recurrent myocardial infarction, and decrease total mortality in clinical trials. Niacin is indicated for the treatment of type IIa, IIb, III, IV, and V hyperlipoproteinemias.
Niacin is thought to inhibit lipoprotein synthesis in the liver and decreases the production of VLDL particles. Nicotinic acid also inhibits the peripheral mobilization of free fatty acids, which results in a decrease in the secretion ofVLDL from the liver. It is the most effective agent at increasing HDL.
Niacin therapy commonly causes skin flushing that is more common with the crystalline formulation. Most people develop a tolerance to this side effect with extended use, but it can be minimized by taking niacin with meals, consuming an aspirin before taking the medication, and gradually increasing the dosage. Gastrointestinal symptoms, including nausea, dyspepsia, flatulence, vomiting, diarrhea, or activation of peptic ulcer, are possible. Hepatotoxicity, hyperuricemia, gout, and hyperglycemia may occur but are more common at higher doses.
Herbals
Red yeast rice products are available OTC and contain lovastatin. The Food and Drug Administration (FDA) issued a warning in 2007 that these products may interact with other medications and have similar toxicities to statins that may not be recognized by consumers. The LDL reduction is minimal. Other OTC products that have demonstrated potential for hyperlipidemia are plant sterols/stanols, supplementary fiber, and immediate-release niacin. Of note, OTC niacin products labeled as “flush-free” contain inositol hexaniacinate, which has not demonstrated significant absorption of nicotinic acid, the active ingredient in niacin products. Therefore, “flush-free” niacin products are generally void of cholesterol benefit.
Triglyceride-Lowering Agents
Fibrates
Fibric acid derivatives or fibrates are indicated for the treatment of type II, IV, and V hyperlipidemia (see Table 8–1). Fibrates decrease TG and increase HDL with variable effects on LDL (see Table 8–7). The primary mechanism of action is peroxisome proliferator-activated receptors or PPAR-a agonism. There also may be subsequent mechanisms (e.g., enhancing lipoprotein lipase activity, inhibiting VLDL synthesis, and reducing cholesterol synthesis). Commercially available fibrates include gemfibrozil (Lopid®), fenofibrate (TriCor®), and fenofibric acid (Trilipix®).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


