Objectives
- Understand the special barriers to absorption of lipids supplied in the diet
- Describe the phases of lipid digestion
- Understand how lipid digestion is facilitated by gastric events
- Define the mechanisms of lipid digestion in the intestinal lumen
- Identify how bile acids and micelles participate in the process of lipid assimilation
- Understand how lipid digestion is facilitated by gastric events
- Describe events at the level of the intestinal epithelium that govern uptake of different classes of lipids
- Understand how the products of lipolysis cross the brush border
- Delineate pathways for lipid processing in the enterocyte
- Describe how chylomicrons are formed and their eventual disposition
- Understand how the products of lipolysis cross the brush border
- Define how lipid digestion and/or absorption can be altered in the setting of disease
General Principles of Lipid Assimilation
 Lipids are defined as organic substances that are hydrophobic, and thus are more soluble in organic solvents (or cell membranes) than in aqueous solutions. Lipids form an important part of most human diets. First, they are denser in calories than either proteins or carbohydrates, increasing the nutritional content of a given meal. Second, several vitamins are lipids (the so-called fat-soluble vitamins). Third, many of the compounds that account for the flavor and aroma of foods are volatile hydrophobic molecules, meaning that lipids serve as an important vehicle to render food palatable. In short, dietary lipids are tasty!
Lipids are defined as organic substances that are hydrophobic, and thus are more soluble in organic solvents (or cell membranes) than in aqueous solutions. Lipids form an important part of most human diets. First, they are denser in calories than either proteins or carbohydrates, increasing the nutritional content of a given meal. Second, several vitamins are lipids (the so-called fat-soluble vitamins). Third, many of the compounds that account for the flavor and aroma of foods are volatile hydrophobic molecules, meaning that lipids serve as an important vehicle to render food palatable. In short, dietary lipids are tasty!
 In the last chapter, we considered the barriers to assimilation of the water-soluble nutrients, carbohydrate, and protein. These molecules are readily soluble in the aqueous environment of the gut lumen, but, following digestion, they require special mechanisms to facilitate their transport across the hydrophobic domain of the enterocyte apical membrane. Conceptually, when considering the assimilation of dietary lipids, the opposite problems pertain. The products of lipid digestion—lipolysis—are, in a large part, readily able to cross cell membranes to allow for absorption into the body. However, lipids are not “at home” in the aqueous milieu of the intestinal contents. Likewise, they must interact with lipolytic enzymes that are themselves soluble proteins. Finally, the products of lipolysis must arrive at the brush border at a sufficient rate to allow for uptake before being propelled along and out of the gut. Systems therefore exist to maintain lipids in suspension in the gut contents with a sufficiently dispersed surface area to allow for lipolysis at the oil–water interface. Additional phase transitions allow for efficient trafficking of lipids to the enterocyte surface, where they can be absorbed.
In the last chapter, we considered the barriers to assimilation of the water-soluble nutrients, carbohydrate, and protein. These molecules are readily soluble in the aqueous environment of the gut lumen, but, following digestion, they require special mechanisms to facilitate their transport across the hydrophobic domain of the enterocyte apical membrane. Conceptually, when considering the assimilation of dietary lipids, the opposite problems pertain. The products of lipid digestion—lipolysis—are, in a large part, readily able to cross cell membranes to allow for absorption into the body. However, lipids are not “at home” in the aqueous milieu of the intestinal contents. Likewise, they must interact with lipolytic enzymes that are themselves soluble proteins. Finally, the products of lipolysis must arrive at the brush border at a sufficient rate to allow for uptake before being propelled along and out of the gut. Systems therefore exist to maintain lipids in suspension in the gut contents with a sufficiently dispersed surface area to allow for lipolysis at the oil–water interface. Additional phase transitions allow for efficient trafficking of lipids to the enterocyte surface, where they can be absorbed.
 Lipids represent a major source of calories in most Western diets, with an average of 120–150 g consumed on a daily basis by a typical adult. Despite their hydrophobicity, the process of lipid assimilation has evolved to be highly efficient, with significant reserve capacity also present in the system. The ready availability of lipid-rich foods in developed countries may therefore be an important contributor to the burgeoning problem of obesity. Indeed, the intestine is also an active participant in lipoprotein metabolism and homeostasis, with implications for the health of the cardiovascular and other body systems. On a daily basis, the intestine is also presented with 40–50 g of endogenous lipid arising from the biliary system.
Lipids represent a major source of calories in most Western diets, with an average of 120–150 g consumed on a daily basis by a typical adult. Despite their hydrophobicity, the process of lipid assimilation has evolved to be highly efficient, with significant reserve capacity also present in the system. The ready availability of lipid-rich foods in developed countries may therefore be an important contributor to the burgeoning problem of obesity. Indeed, the intestine is also an active participant in lipoprotein metabolism and homeostasis, with implications for the health of the cardiovascular and other body systems. On a daily basis, the intestine is also presented with 40–50 g of endogenous lipid arising from the biliary system.
 Lipid in the diet as well as in endogenous pools comprises several distinct molecular classes. This has implications for the absorption of these substrates since greater or lesser degrees of hydrophobicity may govern the precise pathways by which such molecules are taken up by the body. The majority of lipid in the diet is in the form of long-chain triglycerides (i.e., 3 fatty acids with at least 12 carbon atoms each, esterified to glycerol). Phospholipids, which are components of cell membranes, are also significant contributors. Note also that one phospholipid, phosphatidylcholine, is additionally an important constituent of the mixed micelles present in the bile, as we discussed in Chapter 11. Other, more minor sources of dietary lipids include plant sterols (whose absorption may be inefficient) and cholesterol, another membrane constituent that is present in the diets of all except vegans, who consume no meat or dairy products. However, even vegans will encounter cholesterol in the intestinal contents because, like phosphatidylcholine, cholesterol is secreted into the bile. In fact, the endogenous secretion of cholesterol of 1–2 g/day usually exceeds dietary intake of 200–500 mg that is typical of most individuals. The lipid fraction of oral intake may also include hydrophobic xenobiotics and plant waxes.
Lipid in the diet as well as in endogenous pools comprises several distinct molecular classes. This has implications for the absorption of these substrates since greater or lesser degrees of hydrophobicity may govern the precise pathways by which such molecules are taken up by the body. The majority of lipid in the diet is in the form of long-chain triglycerides (i.e., 3 fatty acids with at least 12 carbon atoms each, esterified to glycerol). Phospholipids, which are components of cell membranes, are also significant contributors. Note also that one phospholipid, phosphatidylcholine, is additionally an important constituent of the mixed micelles present in the bile, as we discussed in Chapter 11. Other, more minor sources of dietary lipids include plant sterols (whose absorption may be inefficient) and cholesterol, another membrane constituent that is present in the diets of all except vegans, who consume no meat or dairy products. However, even vegans will encounter cholesterol in the intestinal contents because, like phosphatidylcholine, cholesterol is secreted into the bile. In fact, the endogenous secretion of cholesterol of 1–2 g/day usually exceeds dietary intake of 200–500 mg that is typical of most individuals. The lipid fraction of oral intake may also include hydrophobic xenobiotics and plant waxes.
A special class of dietary lipids is the fat-soluble vitamins. While these are only present in trace amounts, like other vitamins, their absorption is critical for a variety of body processes. The fat-soluble vitamins are A, D, E, and K. Vitamin A (retinoic acid) is an important regulator of gene transcription. Vitamin D regulates calcium absorption by the intestine, and homeostasis of this ion throughout the body. Vitamin E (tocopherol) is a vital antioxidant. Finally, vitamin K is utilized by the liver to catalyze the posttranslational modification of several blood-clotting factors. The fat-soluble vitamins, as a group, have negligible aqueous solubility. Thus, their absorption depends critically on mechanisms designed to enhance their diffusion across aqueous barriers, as will be discussed in more detail later.
Intraluminal Digestion
As for assimilation of protein and carbohydrate, the initial stages of lipid assimilation take place in the intestinal lumen. Luminal events include dispersion of the lipid phase, which is liquid at body temperature, into an emulsion, thereby maximizing the area of the oil–water interface at which lipolysis occurs. Luminal events also include lipolysis, mediated by a series of pancreatic and other enzymes, and uptake of the products of lipolysis into micelles, which can then transfer these molecules to the epithelial surface. Indeed, there is an ordered series of phase transitions that facilitate lipid assimilation. Oil droplets are converted to lamella, vesicular, and liquid crystalline product phases, and finally to micelles that contain the products of lipolysis together with bile acids.
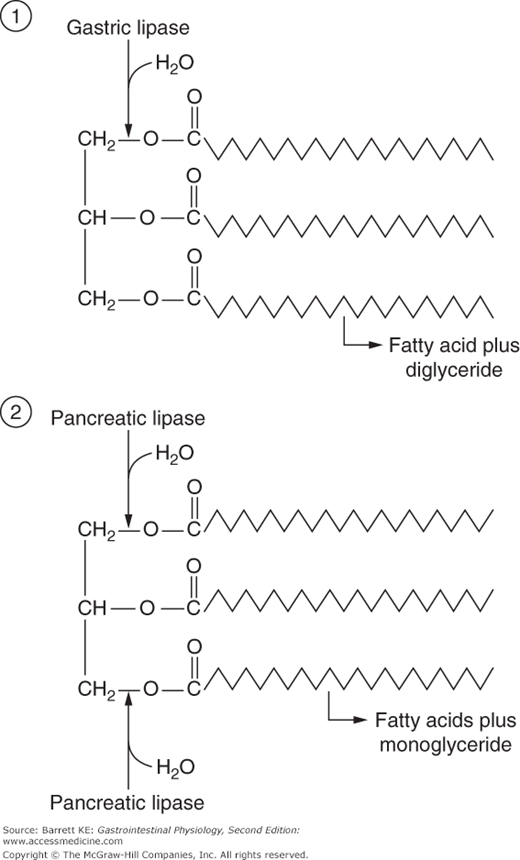
Digestion of the lipid components of the diet begins in the stomach. Gastric peristalsis and mixing patterns provide a shearing action that disperses triglyceride and phospholipids into a fine emulsion. The oil droplets within this emulsion can then be acted upon by gastric lipase, a product of chief cells in the gastric glands that is released in response to neurohumoral triggers coincident with meal intake, and which also promotes gastric acid secretion (see Chapter 3). The lipase binds to the surface of the oil droplets where it can act on triglyceride molecules to generate free fatty acids and diglycerides. However, at the low pH that pertains in the gastric lumen, the fatty acids become protonated and therefore move into the center of oil droplets. Thus, overall, gastric lipolysis is both incomplete, and fails to generate products that are free to diffuse to the mucosal surface where they might be absorbed. In general, 10–30% of overall lipolysis takes place in the stomach in a healthy adult, and gastric digestion of lipids is not essential to their normal uptake. On the other hand, gastric lipolysis may assume a more pronounced role in neonates, where there is a developmental delay in the full expression of pancreatic enzymes, as well as in adults suffering from disease states that impair the production or outflow of pancreatic juice.
Gastric lipase, the enzyme that initiates digestion in the lumen of the stomach, is specialized for activity in the unique conditions that pertain to this segment of the gastrointestinal tract. Most notably, the enzyme displays a pH optimum consistent with that of the gastric contents: 4.0–5.5. The enzyme is also relatively resistant to the action of pepsin, the proteolytic enzyme that is also produced by chief cells. Further, gastric lipase is independent of the presence of any specific cofactors, but is inhibited by bile acids. Finally, gastric lipase acts preferentially to hydrolyze the fatty acid linked to the first position of triglyceride (Figure 16–1) and is subject to end-product inhibition such that gastric lipolysis is largely incomplete from the standpoint that the triglyceride molecule is not fully broken down to its component parts.
The meal moves from the acidic gastric environment to that of the small intestine. As the pH rises secondary to pancreatic, biliary, and duodenal bicarbonate secretion, the fatty acids that were liberated by gastric lipase become ionized and orient themselves to the outside of the oil droplets in the intestinal content. This surrounds the droplet with a layer of ionized fatty acids that serves to stabilize the fat emulsion. Because even long-chain fatty acids have measurable solubility in water, some will dissociate from the droplet and traverse the lumen to meet with the intestinal epithelium. Fatty acids are potent stimuli of cholecystokinin (CCK) release, likely acting via the initial release of the CCK-releasing peptide that we discussed in Chapter 4. Cholecystokinin has a number of actions that are pertinent to lipid digestion and absorption. First, it causes an increase in secretion of pancreatic enzymes. Second, it relaxes the sphincter of Oddi, allowing outflow of the pancreatic juice into the intestinal lumen, and finally, it contracts the gallbladder, providing a bolus of concentrated bile that contains the bile acids needed eventually to dissolve the products of lipolysis in mixed micelles.
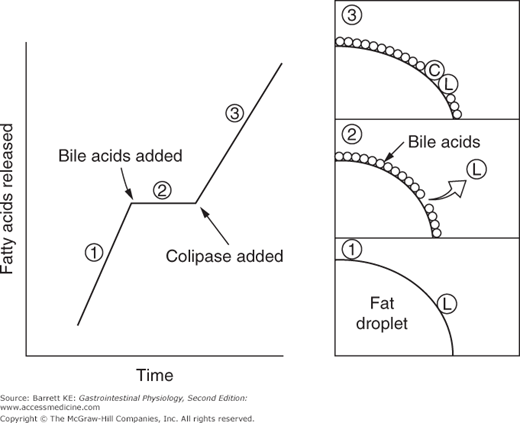
Pancreatic acinar cells secrete a number of proteins that are important in fat digestion (Table 16–1). The first of these is pancreatic lipase. This enzyme is functionally related to the gastric lipase we considered above, but it displays a number of important differences. First, it has a different positional specificity, acting on both the 1 and 3 positions of the glycerol molecule to liberate esterified fatty acids (Figure 16–1). Thus, the products of pancreatic lipase are fatty acids and monoglycerides. Second, it displays a pH optimum in the neutral range, more suited to the conditions that pertain once gastric acid has been neutralized. Indeed, among all of the pancreatic enzymes, lipase is the most susceptible to acid inactivation, meaning that malabsorption of lipid (detected as fat in the stool, or steatorrhea) is often the earliest symptom of pancreatic dysfunction. However, both gastric and pancreatic lipases share the property of being inhibited by bile acids. This is not a major issue in the stomach, which is proximal to the entry of bile. Thus the stomach should contain few bile acids in health. How to solve this conundrum, on the other hand, for pancreatic lipase? The answer lies in the presence of a second product of pancreatic acinar cells, colipase. Colipase is synthesized as an inactive precursor (procolipase) that is secreted in approximately equimolar amounts with lipase, and is activated by proteolytic cleavage when it reaches the intestinal lumen. Colipase is capable of binding to both bile acids and lipase, which stabilizes the presence of lipase on the surface of luminal oil droplets. The significance of this interaction is shown in Figure 16–2, which depicts the rate of lipolysis of an oil emulsion under various experimental conditions. If lipase alone is present, it adsorbs to the surface of the oil droplets and generates free fatty acids. With addition of bile acids, however, these array themselves on the surface of the oil droplets and displace lipase, halting its enzymatic activity. However, if colipase is also present, the binding specificities of this molecule can therefore anchor lipase to the oil droplet, and thus its lipolytic action is restored.
| Protein | Source | Activity | Comments |
|---|---|---|---|
| Pancreatic lipase | Pancreatic acinar cells | Hydrolyzes 1 and 3 positions of triglyceride | Inhibited by bile acids |
| Colipase | Proform secreted by pancreatic acinar cells | Cofactor for lipase | Binds to lipase and to bile acids |
| Secretory phospholipase A2 | Proform secreted by pancreatic acinar cells | Hydrolyzes fatty acid in 2 position of phospholipids | Requires calcium for activity |
| Cholesterol esterase | Pancreatic acinar cells | Broad substrate specificity—cholesterol and vitamin esters; 1, 2, and 3 positions of triglyceride | Requires bile acids for activity |
| Breast milk lipase | Mammary gland | Related to cholesterol esterase | Important in neonates |