Lichtenstein Tension-Free Hernioplasty
Parviz K. Amid
David C. Chen
Weakening of the abdominal wall tissue as one of the causes of inguinal hernias was suspected by Cooper as far back as 1800. The matter was emphasized again in 1922 by Harrison when he wondered why a significant number of men develope hernias at age 50 to 60, years after their active life is over. Need for prosthetic reinforcement of weakened abdominal wall tissue was recognized by Billroth, musing “If only the proper material could be created to artificially produce tissue of density and toughness of fascia and tendon, the secret of the radical cure for hernia would be discovered.” However, early generations of prostheses resulted in disastrous complications from rejection and infection. It was not until the introduction of polypropylene mesh by Usher in 1959 that Billroth’s dream was realized.
With the necessity of prosthesis for the repair of inguinal hernia in mind, and focusing on the principle of “no tension” (considered one of the great principles of surgery by Halstead), the Lichtenstein group popularized routine use of mesh in 1984 and coined the term “tension-free hernioplasty.” During the rapid evolution of hernia surgery throughout the past decade, it was left to Nyhus to remove the previous generation’s fear of infection and rejection of prosthesis, which he did when he stated in 1989, “My concerns relative to the potentially increased incidents of infection or rejection of polypropylene mesh have not been warranted to date.”
Today, understanding the role of protease–antiprotease imbalance in the pathogenesis of groin hernias has led to a new grasp of the pathology of groin hernias and the causes of their surgical failure. There is morphologic and biochemical evidence that adult male inguinal hernias are associated with an altered collagen type I to type III ratio. These changes lead to weakening of the fibroconnective tissue of the groin and development of inguinal hernias. To use this already defective tissue, especially under tension, is a violation of the most basic principles of surgery.
In tension-free hernioplasty, instead of suturing anatomic structures that are not in apposition, the entire inguinal floor is reinforced by insertion of a sheet of mesh. The prosthesis that is placed between the transversalis fascia and the external oblique aponeurosis extends well beyond Hesselbach’s triangle in order to provide sufficient mesh–tissue interface. On increased intra-abdominal pressure, the external oblique aponeurosis applies counterpressure to the mesh, thus using the intra-abdominal pressure in favor of the repair. The procedure addresses both issues of hazardous suture line tension and metabolic causation of inguinal hernias. The operation is therefore therapeutic as well as prophylactic; thus it repairs and protects the entire susceptible region of the groin to herniation from all future metabolic and mechanical adverse effects.
The procedure is performed under local anesthesia, which is our preferred choice for all reducible adult inguinal hernias. It is safe, simple, effective, economical, and without any side effects such as nausea, vomiting, and urinary retention. Furthermore, local anesthesia administered prior to making the incision produces a prolonged analgesic effect via inhibition of the buildup of local nociceptive molecules.
Technique of Anesthesia
Several safe and effective anesthetic agents are currently available. Our choice, however, is a 50:50 mixture of 1% lidocaine (Xylocaine) and 0.5% bupivacaine (Marcaine), with 1/200,000 epinephrine. An average of 45 mL of this mixture is usually sufficient for a unilateral hernia repair and is administered in the following fashion.
Subdermal Infiltration
Approximately 5 mL of the mixture is infiltrated along the line of the incision with a 1.5-in.-long, 25-gauge needle inserted into the subdermal tissue parallel to the surface of the skin. Infiltration continues as the needle is advanced. Movement of the needle reduces the likelihood of intravascular infusion of the drugs because even if the needle penetrates a blood vessel, the tip will not remain in the vessel long enough to deliver a substantial amount of the anesthetic agent intravenously. This step blocks the subdermal nerve endings and reduces the discomfort of the intradermal infiltration, which is the most uncomfortable stage of local anesthesia.
Intradermal Injection (Creation of the Skin Wheal)
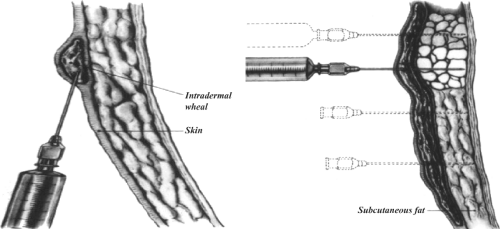
The needle in the subdermal plane is withdrawn slowly until the tip of the needle reaches the intradermal level. Without extracting the needle completely, the dermis is infiltrated by slow injection of approximately 3 mL of the mixture along the line of the incision (Fig. 1, left).
Deep Subcutaneous Injection
A total of 10 mL of the mixture is injected deep into the subcutaneous adipose tissue through vertical insertions of the needle (perpendicular to the skin surface) 2 cm apart. Again, injections are continued as the needle is kept moving to reduce the risk of intravascular infusion (Fig. 1).
Subaponeurotic Injection
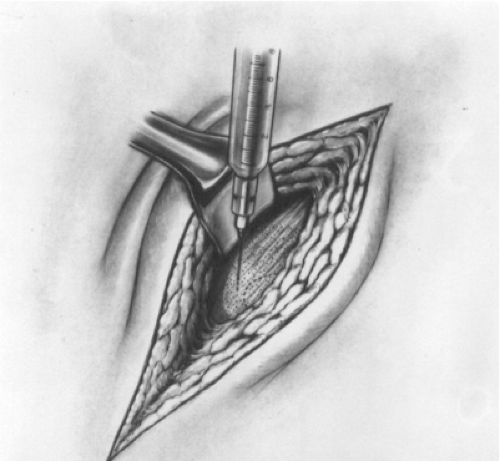
Approximately 10 mL of the anesthetic mixture is injected immediately underneath the aponeurosis of the external oblique muscle through a window created in the subcutaneous fat at the lateral corner of the incision (Fig. 2). This injection floods the enclosed inguinal canal and anesthetizes all three major nerves in the
region while the remaining subcutaneous fat is incised.
region while the remaining subcutaneous fat is incised.
It also separates the external oblique aponeurosis from the underlying ilioinguinal nerve, reducing the likelihood of injuring the nerve when the external oblique aponeurosis is incised.
Occasionally it is necessary to infiltrate a few milliliters of the mixture at the level of the pubic tubercle, around the neck and inside the indirect hernia sac, to achieve complete local anesthesia.
Analgesic effect of the local anesthesia can be further prolonged by splashing 10 mL of the mixture into the inguinal canal before closure of the external oblique aponeurosis and in the subcutaneous space before skin closure.
Sedative drugs given by the surgeon—or preferably by an anesthetist as “conscious sedation” via infusion of rapid, short-acting, amnesic, and anxiolytic agents such as propofol—reduce the patient’s anxiety. This also reduces the amount of local anesthetic agents required, particularly for bilateral inguinal hernia repair. Epidural anesthesia is preferred for repair of nonreducible inguinal hernias.
Technique of the Operation
A 5- to 6-cm skin incision, which starts from the pubic tubercle and extends laterally within the Langer line, gives an excellent exposure of the pubic tubercle and the internal ring. After skin incision, the external oblique aponeurosis is opened and its lower leaf is freed from the spermatic cord. The upper leaf of the external oblique is then freed from the underlying internal oblique muscle until the internal oblique aponeurosis is exposed.
The anatomic cleavage between these two layers is avascular and the dissection can be done rapidly and atraumatically. High separation of these layers has a dual benefit because it visualizes the iliohypogastric nerve and internal oblique aponeurosis and creates ample space for insertion of a sufficiently wide sheet of mesh that can overlap the internal oblique well above the upper margin of the inguinal floor. The cord with its cremaster covering is separated from the floor of the inguinal canal and the pubic bone for a distance of approximately 2 cm beyond the pubic tubercle.
The anatomic plane between the cremasteric muscle and attachment of rectus sheath to the pubic bone is avascular, so there is no risk of damaging the testicular blood flow. When lifting the cord, care should be taken to include the ilioinguinal nerve, the easily visible blue external spermatic vein (the blue line), and the genital nerve with the cord. This ensures that the genital nerve, which is always in juxtaposition to the external spermatic vessels, is preserved.
To explore the internal ring, for indirect hernia sacs, the cremasteric sheath is incised longitudinally at the level of the deep ring to access the cremasteric compartment. Not removing the cremasteric muscle prevents the testicle from hanging low and also prevents dysfunction of the cremasteric muscle, which may lead to dysejaculation. Complete stripping and resection of the cremasteric fibers is unnecessary and can result in direct exposure of the genital nerve, vas deferens, and paravasal nerves to the mesh, resulting in chronic groin and testicular pain.
Indirect hernial sacs are freed from the cord to a point beyond the neck of the sac and are inverted into the properitoneal space without ligation. Because of mechanical pressure and ischemic changes, ligation of the highly innervated peritoneal sac is a major cause of postoperative pain. It has been shown that nonligation of the indirect hernia sac does not increase the chance of recurrence. To minimize the risk of postoperative ischemic orchitis, complete nonsliding scrotal hernia sacs are transected at the midpoint of the canal, leaving the distal section in place. However, the anterior wall of the distal sac is incised to prevent postoperative hydrocele formation.
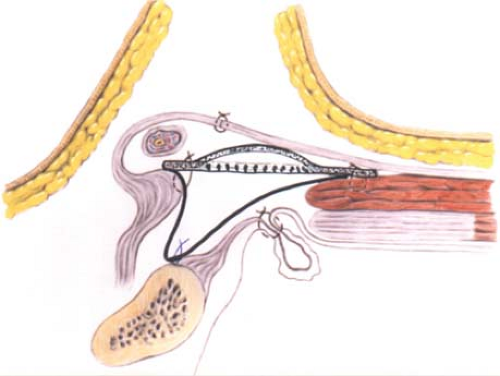
In the event of direct hernias, if large, the direct sacs are inverted with an absorbable suture (Fig. 3). A thorough exploration of the groin is necessary to rule out the coexisting intraparietal (interstitial), low-lying spigelian or femoral hernias. The femoral ring is routinely evaluated via the space of Bogros through a small opening in the canal floor. A sheet of 8 × 16 cm of mesh is used. We prefer monofilament macroporous polypropylene meshes because their monofilament structure does not perpetuate or harbor infection. The medial corner of the mesh is tailored to its standard shape, which resembles the tracing of a footprint, with a lower sharper angle to fit into the angle between the inguinal ligament and the rectus sheath and an upper wider angle to spread over the rectus sheath (Fig. 4). With the cord retracted upward, the sharper corner of the mesh is secured with a nonabsorbable monofilament suture to the insertion of the rectus sheath to the pubic bone overlapping the bone by 1 to 2 cm
(Fig. 5). This is a crucial step in the repair because failure to cover this bone with the mesh can result in recurrence of the hernia. The periosteum of the bone is avoided. This suture is continued (as a continuous suture with up to four passages) to attach the lower edge of the patch to the inguinal ligament up to a point just lateral to the internal ring (Fig. 5). Suturing the mesh beyond this point is unnecessary and could injure the femoral nerve. If there is a concurrent femoral hernia, it can be fixed using my maneuver in which the mesh is also sutured to the Cooper’s ligament 1 to 2 cm below its suture line with the inguinal ligament in order to close the femoral ring (Fig. 3, solid black line). Alternatively, the mesh can be tailored to have a triangular extension from its lower edge. The long side of the dropped-down triangle is sutured to the Cooper’s ligament and the body of the mesh is sutured to the inguinal ligament along the solid line (Fig. 6).
(Fig. 5). This is a crucial step in the repair because failure to cover this bone with the mesh can result in recurrence of the hernia. The periosteum of the bone is avoided. This suture is continued (as a continuous suture with up to four passages) to attach the lower edge of the patch to the inguinal ligament up to a point just lateral to the internal ring (Fig. 5). Suturing the mesh beyond this point is unnecessary and could injure the femoral nerve. If there is a concurrent femoral hernia, it can be fixed using my maneuver in which the mesh is also sutured to the Cooper’s ligament 1 to 2 cm below its suture line with the inguinal ligament in order to close the femoral ring (Fig. 3, solid black line). Alternatively, the mesh can be tailored to have a triangular extension from its lower edge. The long side of the dropped-down triangle is sutured to the Cooper’s ligament and the body of the mesh is sutured to the inguinal ligament along the solid line (Fig. 6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree