LEGIONELLA PNEUMOPHILA AND OTHER LEGIONELLAE
A widely publicized outbreak of pneumonia in persons attending an American Legion convention in Philadelphia in 1976 prompted investigations that defined Legionella pneumophila and the legionellae. Other outbreaks of respiratory illness caused by related organisms since 1947 have been diagnosed retrospectively. Several dozen species of Legionella exist, some with multiple serogroups. L pneumophila is the major cause of disease in humans; Legionella micdadei and a few other species sometimes cause pneumonia. The other legionellae are rarely isolated from patients or have been isolated only from the environment.
L pneumophila is the prototype bacterium of the group. Legionellae of primary medical importance are listed in Table 22-1.
| Species | Pneumonia | Pontiac Fever |
|---|---|---|
| Legionella pneumophila | + | Serogroups 1 and 6 |
| Legionella micdadei | + | |
| Legionella gormanii | + | |
| Legionella dumoffii | + | |
| Legionella bozemanae | + | |
| Legionella longbeachae | + | |
| Legionella wadsworthii | + | |
| Legionella jordanis | + | |
| Legionella feeleii | + | + |
| Legionella oakridgensis | + | |
| Legionella birminghamensis | + | |
| Legionella cincinnatiensis | + | |
| Legionella hackeliae | + | |
| Legionella lansingensis | + | |
| Legionella parisiensis | + | |
| Legionella sainthelensi | + | |
| Legionella tusconensis | + |
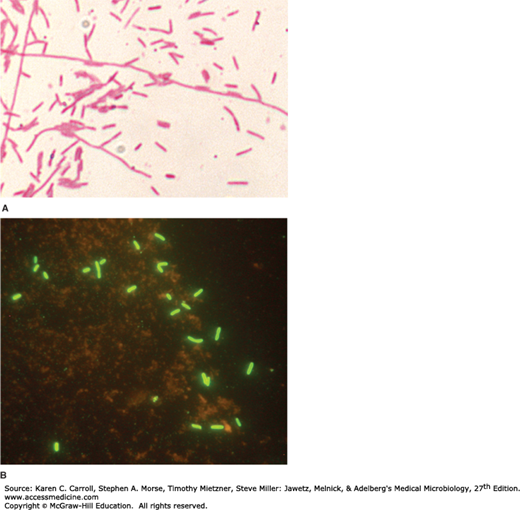
Legionellae are fastidious, aerobic gram-negative bacteria that are 0.5–1 μm wide and 2–50 μm long (Figure 22-1). They often stain poorly by Gram’s method and are not easily seen in stains of clinical specimens. Gram-stained smears should be made for suspect Legionella growth on agar media. Basic fuchsin (0.1%) should be used as the counterstain because safranin stains the bacteria very poorly. Silver stains, such as Warthin-Starry and Dieterle, can be used to detect legionellae in embedded tissues. Of note, L micdadei may be acid-fast stain positive.
FIGURE 22-1
A: Gram stain of a Legionella pneumophila; the legionellae stain faintly with basic fuchsin and poorly with safranin. Original magnification ×1000. (Courtesy CDC Public Health Image Library.) B: Direct fluorescent antibody stain of Legionella of mixed species using antibodies against legionellae genus antigens conjugated with fluorescein. Original magnification ×1000. (Courtesy R Nadarajah.)
Legionellae can be grown on complex media such as buffered charcoal yeast extract agar with α-ketoglutarate, L-cysteine, and iron (BCYE) at a pH of 6.9, temperature of 35°C, and 90% humidity. Antibiotics can be added to make the medium selective for Legionella species. The charcoal acts as a detoxifying agent. Legionellae grow slowly; visible colonies are usually present after 3 days of incubation. Colonies that appear after overnight incubation are not Legionella species. Colonies are round or flat with entire edges. They vary in color from colorless to iridescent pink or blue and are translucent or speckled. Variation in colony morphology is common, and the colonies may rapidly lose their color and speckles. Many other genera of bacteria grow on BCYE medium and must be differentiated from Legionella by Gram staining and other tests.
Suspicious colonies require definitive identification by methods other than biochemical assessment since legionellae are biochemically inert. Confirmatory tests include direct fluorescent antibody tests, 16SrRNA gene sequencing, and the use of matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS).
The legionellae are catalase positive. L pneumophila is oxidase positive; the other legionellae are variable in oxidase activity. L pneumophila hydrolyzes hippurate; the other legionellae do not. Most legionellae produce gelatinase and β-lactamase; L micdadei produces neither gelatinase nor β-lactamase.
Antigenic specificity of L pneumophila is thought to be attributable to complex antigenic structures. There are at least 16 serogroups of L pneumophila; serogroup 1 was the cause of the 1976 outbreak of Legionnaires’ disease and remains the most common serogroup isolated from humans. Legionella species cannot be identified by serogrouping alone because there is cross-reactive antigenicity among different species. Occasionally, other gram-negative bacteria may also cross-react with L pneumophila antisera.
The legionellae produce distinctive 14- to 17-carbon branched-chain fatty acids. Gas-liquid chromatography can be used to help characterize and determine the species of legionellae.
The legionellae make proteases, phosphatase, lipase, DNase, and RNase. A major secretory protein, a metalloprotease, has hemolytic and cytotoxic activity; however, this protein has not been shown to be a required virulence factor.
Legionellae are ubiquitous in warm, moist environments. They are found in lakes, streams, and other bodies of water. They can multiply in free-living amebas and can coexist with them in biofilms (see Epidemiology and Control). Infection of debilitated or immunocompromised humans commonly follows inhalation of the bacteria from aerosols generated from contaminated air-conditioning systems, shower heads, and similar water sources. L pneumophila usually produces a lobar, segmental, or patchy pulmonary infiltration. Histologically, the appearance is similar to that produced by many other bacterial pathogens. Acute pneumonia involving the alveoli is present with a dense intra-alveolar exudate of macrophages, polymorphonuclear leukocytes, red blood cells, and proteinaceous material. Most of the legionellae in the lesions are within phagocytic cells. There is little interstitial infiltration and little or no inflammation of the bronchioles and upper airways.
Knowledge of the pathogenesis of L pneumophila infection comes from study of isolated cells from humans and from study of susceptible animals such as guinea pigs.
L pneumophila readily enters and grows within human alveolar macrophages and monocytes and is not effectively killed by polymorphonuclear leukocytes. Legionella species do not require opsonization by C3b or antibody to enter macrophages. A virulence factor important for macrophage invasion is the Mip protein, which promotes adherence and phagocytosis. Inside the cell, the individual bacteria are contained within phagosomal vacuoles (Legionella-containing vacuole, LCV), but the defense mechanisms of the macrophages stop at that point. Instead, the LCV fails to fuse with lysosomal granules. The phagocyte oxidative metabolic burst is reduced. The LCV does not acidify as much as phagosomes containing other ingested particles. Ribosomes, mitochondria, and small vesicles accumulate around LCVs, preventing recognition by the cellular immune system. In addition to this process, phagosome survival and organism replication are facilitated by elaboration of a type IV secretion system call Dot/Icm which is essential for L pneumophila virulence. The bacteria multiply within the vacuoles until they are numerous, the cells are destroyed, the bacteria are released, and infection of other macrophages then occurs. The presence of iron (transferrin iron) is also essential for the process of intracellular growth of the bacteria.
Asymptomatic infection is common in all age groups, as shown by elevated titers of specific antibodies. The incidence of clinically significant disease is highest in men after age 55 years. Disease in children has been reported but remains rare. Factors associated with high risk include smoking, alcohol misuse, diabetes mellitus, chronic bronchitis and emphysema or cardiovascular disease, steroid and other immunosuppressive treatment (as in renal transplantation), cancer chemotherapy, and, more recently, as a complication of anti–tumor necrosis factor (TNF)-α therapy, especially infliximab or adalimumab. When pneumonia occurs in patients with these risk factors, Legionella should be investigated as the cause.
Infection may result in nondescript febrile illness of short duration or in a severe, rapidly progressive illness with high fever, chills, malaise, nonproductive cough, hypoxia, diarrhea, and delirium. Chest radiography reveals patchy, often multilobar consolidation. Immunocompromised patients may develop cavitary pneumonia and pleural effusions. There may be leukocytosis, hyponatremia, hematuria (and even renal failure), or abnormal liver function. During some outbreaks, the mortality rate has reached 10%. The diagnosis is based on the clinical picture and exclusion of other causes of pneumonia by laboratory tests. Demonstration of Legionella species in clinical specimens can rapidly yield a specific diagnosis. The Legionella urinary antigen test may be used early in the course of infection with L pneumophila serogroup 1 and is very helpful when the result is positive. The diagnosis can also be made by culture for Legionella species or by serologic tests, but results of these tests are often delayed beyond the time when specific therapy must be started.
L pneumophila also produces a disease called “Pontiac fever” after the clinical syndrome that occurred in an outbreak in Michigan. The syndrome is characterized by fever and chills, myalgia, malaise, and headache that develop over 6–12 hours and persist for 2–5 days. Dizziness, photophobia, neck stiffness, and confusion also occur. Respiratory symptoms are much less prominent in patients with Pontiac fever than in those with Legionnaires’ disease and include mild cough and sore throat. This illness is self-limited and does not require treatment with antibiotics.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree