I. NORMAL ANATOMY
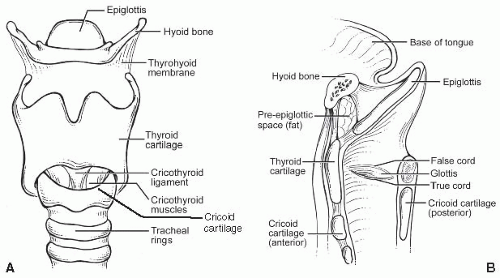
A. Macroscopic/gross. The larynx is a unique organ designed to produce phonation by modulation of the respiratory airstream. It is composed of several cartilaginous structures: the thyroid, cricoid, and arytenoid cartilages and the epiglottis. The hyoid bone sits above and is connected to the larynx by the thyrohyoid membrane (Fig. 2.1). The thyroid (and lesser so, cricoid cartilage) ossifies in adults. In functional terms, the larynx is divided into three subsites. The glottis includes the true vocal folds or cords, below, and the false folds or cords, above. The space between them is called the ventricle, and its deeper recess, the saccule. The true cords, with a hypocellular stroma called Reinke’s space, are designed to vibrate for phonation, and the ventricle amplifies this further. The cords are manipulated by muscles that attach to and move the arytenoid cartilages, which sit at the posterior aspect of the vocal folds. The larynx can be divided into three compartments for tumor management and staging purposes: the supraglottis, glottis, and subglottis. The supraglottis includes the epiglottis, aryepiglottic folds, false cords, and ventricle. Glottis refers to the vocal cords from the edge of the ventricle to the free edge of the vocal cord. Subglottis refers to the area from the free edge of the vocal fold to the inferior border of the cricoid cartilage but has a slightly different definition for American Joint Committee on Cancer (AJCC) staging (see later).
B. Microscopic. The larynx is covered by a mixture of squamous and pseudostratified ciliated columnar (respiratory-type) epithelium. In smokers, however, often the entire endolarynx is covered by squamous epithelium. The true cords themselves are always covered by squamous epithelium. They contain a lamina propria area called Reinke’s space, which lies between the epithelium and the vocal ligament and which consists of loose connective tissue with few capillaries, no lymphatics, and sparse seromucinous glands (e-Fig. 2.1).* The false cords and ventricle are typically lined by respiratory-type epithelium.
II. GROSS EXAMINATION, TISSUE SAMPLING, AND HISTOLOGIC SLIDE PREPARATION
A. Endoscopic biopsies. The majority of specimens from this region consist of endoscopic forcep biopsies. The tissue samples should be placed immediately into 10% buffered formalin or other appropriate fixative. These should undergo gross examination and description documenting the exact number of pieces present and their size, and then be entirely submitted with three levels cut from each paraffin block for hematoxylin and eosin (H&E) examination.
B. Resections
1. Partial. These are widely variable specimens depending on the location of the tumor. Standard procedures include vertical hemilaryngectomy and supraglottic or supracricoid laryngectomy. As a generalization, these specimens need to be oriented, the soft tissue margins inked, and margins demonstrated by shave or radial section followed by sectioning of the tumor relative to cartilage/bone and soft tissue margins. The use of either shave or radial sections depends on the nature of the specimen. If the tumor is relatively distant from a margin, 1- to 2-mm shave sections are preferred. If the tumor approximates a margin to <1 to 2 mm, radial sections are taken.
Partial resections with a CO2 laser under an operating microscope are becoming more common because of their low morbidity. Because an inherent part of this procedure is to cut into the tumor or to remove tumor in more than one piece, surgeons ink the individual pieces themselves, as they alone know what constitutes the true margin. In the pathology lab, the pieces are measured, described, and submitted entirely in sections perpendicular to the ink.
2. Total. For years, total laryngectomy has been the standard operation for malignancy. However, partial resections with preservation of function are increasingly common. Total laryngectomy is used most often now as salvage therapy for recurrences after partial surgery or after definitive radiation and chemotherapy. Occasionally, patients present with progressive disease that makes total laryngectomy necessary as the initial surgery (e-Fig. 2.2).
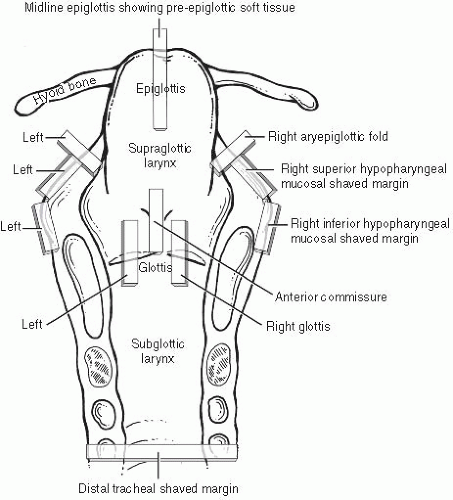
The usual approach to grossing a total laryngectomy is to initially ink the peripheral nonmucosal soft tissue margins and then open the larynx by a posterior vertical midline cut with scissors, propping it wide open with a small stick or portion of a wooden swab. After overnight formalin fixation, the specimen is ready for prosection. After orientation and measurement in three dimensions, the tumor is measured and described, specifically noting what structures are involved. The specimen typically includes the entire larynx and cartilages, small portions of hypopharyngeal mucosa bilaterally adjacent to the aryepiglottic folds, and the hyoid bone anterosuperiorly. Standard sections (Fig. 2.2) are taken as follows. (i) Margins: shaved inferior tracheal ring; shaved right and left hypopharyngeal mucosa; shaved postcricoid soft tissue; radial sections demonstrating anterior, anterolateral, and base of tongue inked soft tissue margins. (ii) Soft tissue/mucosa: bilateral vertical glottis (including both true and false cords); vertical anterior commissure; vertical midline epiglottis showing pre-epiglottic soft tissue space; bilateral aryepiglottic folds; four sections of tumor if not included in the previous sections. (iii) Cartilage/bone: vertical sections showing tumor and thyroid cartilage (at closest or at sites of gross invasion); sections of right and left wings of the hyoid bone (where closest to or involved by tumor). Typically, all soft tissue and margin sections are taken first, and the entire specimen is
bisected in the midline vertically and decalcified in toto overnight followed by cartilage and/or bone section acquisition.
C. Frozen sections. These are a critical element of surgical therapy for tumors of the head and neck region. Although practices vary, most institutions have margins taken as small pieces by the surgeon from the periphery of the surgical defect after the tumor has been removed. Because laryngeal resections are quite variable, the sites where frozen sections are taken are not standard; with total laryngectomies, sometimes no frozen sections are clinically necessary. The surgeon may sample the tumor or suspicious sites to confirm and/or map the tumor, and then take margins from the area of closest approach after resection of it. The tissue pieces are submitted individually to pathology in saline and frozen in their entirety, with two (and at our institution, three) H&E slides generated at representative levels. It is critical to obtain sections that represent the entire tissue submitted so that small foci of tumor are not missed by “sampling error” (i.e., where tiny foci of tumor are not present on frozen section slides but are seen on permanent sections, due to not “sampling” the tissue well at the time of the frozen). This is best done by making sure that the second and third sections
are taken from deep into the tissue. The pieces should be evaluated grossly for mucosa—typically shiny and pink-tan on one surface of the tissue; if present, the specimen should be oriented to demonstrate this surface on one edge of the section with the submucosa below. Additional sections should be cut if needed to assure that two or three quality sections are obtained.
The tissue that remains after frozen section is submitted for evaluation by permanent sections. This process can help resolve a number of issues from frozen section including freezing and cautery artifact, amount of tumor represented, and orientation or embedding issues. The margins of the main resection specimen are also evaluated throughout its entirety because the separate frozen section specimens are small and almost never cover the entire margin of a resection. The final margin status is then a conglomerate of all three sources: frozen section slides, permanent slides of the frozen tissue, and the margins of the specimen itself.
III. DIAGNOSTIC FEATURES OF COMMON DISEASES
A. Inflammation and infection. Inflammation of the larynx (laryngitis) is quite common clinically, and can be divided into acute and chronic forms, which are variable by age. Laryngitis rarely necessitates tissue biopsy for pathologic evaluation. Infections can be caused by a myriad of agents including viruses, bacteria, fungi, and parasites. The pathologist must be alert to the possibility of infection, and the immune status of the patient is helpful information, as many of these patients will be immunocompromised.
Inflammation is essentially never present in the normal larynx, so the presence of inflammatory cells is a diagnostic clue. Depending on the organism, the inflammation can take a number of different forms, almost all of which are typical for the type of organism when it presents in other locations. Examples of some of the major infections include cytomegalovirus, herpes simplex virus, tuberculosis, rhinoscleroma (Klebsiella rhinoscleromatis), candidiasis, histoplasmosis, blastomycosis, cryptococcosis, coccidiomycosis, or rhinosporidiosis (Rhinosporidium seeberi). The resulting inflammation may cause mucosal ulceration, acute and chronic inflammation, necrosis, or granulomas. Special stains (such as Gomori’s methenamine silver (GMS), acid-fast bacillus (AFB), or periodic acid-Schiff (PAS)) should be utilized to look for, and characterize, organisms.
B. Non-neoplastic lesions
1. Traumatic
a. Vocal cord nodules and polyps. These are non-neoplastic degenerative stromal lesions of Reinke’s space that are usually related to trauma due to misuse or vocal excess. As such, they have been referred to as singer’s or screamer’s nodules. They are more common in women and are commonly bilateral, characteristically occurring at the junction of the anterior and middle one-third of the vocal cord, as this is the point of maximal vibration during phonation. Macroscopically, they appear as gray or white broad-based nodules or polyps (e-Fig. 2.3). Microscopically, they consist of squamous mucosa with or without hyperkeratosis, are only rarely ulcerated, and overlie a sparsely cellular myxoid, edematous, fibrous, fibrinous, or vascular stroma. The myxoid appearance is most common and it may demonstrate small cystic spaces (e-Figs. 2.4 and 2.5). So-called vocal cord polyps are histologically identical but clinically present unilaterally, and in men and smokers with more regularity.
b. Contact ulcer. Also referred to clinically as contact granuloma or just granuloma, these occur on the vocal process of the arytenoids classically as a result of forceful vocalization in individuals who must affect a low, deep, forceful voice. However, they also may result after endotracheal intubation or as a result of gastroesophageal reflux disease. They are more common in men, can be unilateral or bilateral, and present as polypoid lesions.
Microscopically, they are essentially granulation tissue polyps with an ulcerated mucosa and a stroma containing abundant small vessels in a haphazard configuration with plump endothelial cells (e-Fig. 2.6). The stroma may be rich in lymphocytes, plasma cells, neutrophils, or histiocytes (sometimes including giant cells).
2. Cysts. Laryngeal cysts may be divided into three categories: (i) ductal cysts, (ii) laryngoceles, and (iii) saccular cysts. All are cured by simple excision.
a. Ductal cysts. These are the most common and result from obstruction of a minor salivary gland duct. Ductal cysts are typically small “bumps” on endoscopy and have a predilection for the cords, ventricle, aryepiglottic folds, and epiglottis. The cyst lining may be squamous or oncocytic.
b. Laryngocele. A laryngocele is an asymptomatic dilatation of the saccule (the deep aspect of the ventricle). It may remain internal and manifest as a supraglottic mucosal bulge, or may herniate above the thyroid cartilage to project externally into the neck soft tissue and present as a neck mass. Microscopically, it is lined by respiratory-type mucosa (e-Fig. 2.7).
c. Saccular cyst. This represents a mucin-filled dilatation of the saccule, either developmental or acquired. It is also typically lined by respiratory-type mucosa, but the lining can be squamous or oncocytic on occasion. It is easily confused with a branchial cleft cyst.
3. Metabolic
a. Amyloidosis. The larynx is a well-established, although infrequent, site of localized amyloidosis. It most commonly involves the false cord, followed by the true cord and ventricle. A subset of patients has multifocal disease, with approximately one-third having tracheal disease as well. The majority of patients have only localized disease, but systemic disease also occurs in a subset of patients. All patients should have a workup to rule out systemic amyloidosis with or without an associated plasma cell dyscrasia. The amyloid in localized laryngotracheal amyloidosis is of the AL (or immunoglobulin light chain type) type. It usually macroscopically presents as a polypoid nodule covered by intact mucosa. Microscopically, it consists of sheets and nodular masses of amorphous, hypocellular eosinophilic material in the stroma (e-Fig. 2.8), in blood vessel walls, and in the basement membranes of mucoserous glands. The diagnosis is confirmed by Congo red special staining with “apple-green” birefringence on polarized microscopy. Thioflavin T staining with examination under fluorescence microscopy is also sometimes used.
C. Neoplastic lesions. The World Health Organization (WHO) classification of tumors of the larynx, hypopharynx, and trachea is listed in Table 2.1.
1. Benign
a. Squamous papillomas are squamous proliferations caused by human papilloma virus (HPV) and are the most common benign tumors of the larynx. They are most common in the larynx, but also occur in the trachea and bronchi. They also occur occasionally in the oral cavity and pharynx. There are two separate clinical settings: juvenile or juvenile-onset laryngeal papillomatosis (JOLP) and adult (or adult-onset) laryngeal papillomatosis (AOLP). Juvenile papillomas most often begin before the age of 5 years and are much more likely than adult papillomas to be multifocal and to have an associated clinical impact. In a significant minority of patients, carpeting of the larynx occurs, requiring repeated laser excisions and occasionally tracheostomy or even laryngectomy for control and airway management. Papillomas tend to recur rapidly, but the disease severity usually regresses in early adulthood. In adult papillomas, the peak age is between 20 and 40 years, disease is usually unifocal or limited, and even when multifocal, it is less aggressive. Only occasionally does it present as multifocal disease that recurs after excision.
TABLE 2.1 World Health Organization Histological Classification of Tumors of the Hypopharynx, Larynx, and Trachea
Malignant epithelial tumors
Squamous cell carcinoma
Verrucous carcinoma
Papillary squamous cell carcinoma
Basaloid squamous cell carcinoma
Spindle cell carcinoma
Adenosquamous carcinoma
Acantholytic squamous cell carcinoma
Lymphoepithelial carcinoma
Giant cell carcinoma
Malignant salivary gland-type carcinomas
Adenoid cystic carcinoma
Mucoepidermoid carcinoma
Neuroendocrine tumors
Typical carcinoid
Atypical carcinoid
Small cell carcinoma, neuroendocrine type
Combined small cell carcinoma, neuroendocrine type
Benign epithelial tumors
Papilloma
Papillomatosis
Salivary gland-type adenomas
Pleomorphic adenoma
Oncocytic papillary cystadenoma
Soft tissue tumors
Malignant tumors
Fibrosarcoma
Malignant fibrous histiocytoma
Liposarcoma
Leiomyosarcoma
Rhabdomyosarcoma
Angiosarcoma
Kaposi sarcoma
Malignant peripheral nerve sheath tumor
Synovial sarcoma
Borderline tumors/low malignant potential
Inflammatory myofibroblastic tumor
Benign tumors
Schwannoma
Neurofibroma
Lipoma
Leiomyoma
Rhabdomyoma
Hemangioma
Lymphangioma
Granular cell tumor
Hematolymphoid tumors
Tumors of bone and cartilage
Chondrosarcoma
Osteosarcoma
Chondroma
Giant cell tumor
Mucosal malignant melanoma
Secondary tumors
From: Barnes L, Eveson J, Reichart P, Sidransky D, eds. World Health Organization Classification of Tumours. Pathology and Genetics. Head and Neck Tumours. Lyon: IARC Press; 2005. Used with permission.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Larynx
Larynx
James S. Lewis Jr.