ANATOMY
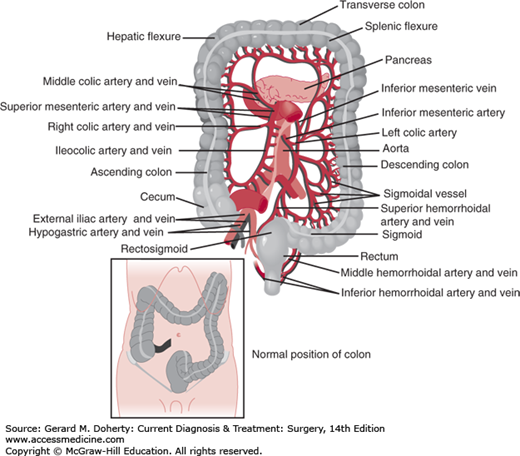
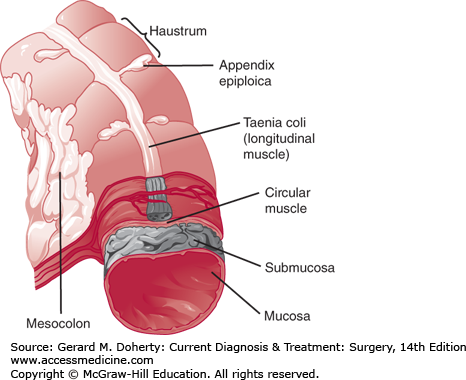
The colon begins at the ileocecal valve and ends at the rectum, spanning 140 cm (5 feet) in length. The colon has both intraperitoneal and retroperitoneal components. The cecum, ascending, and descending colon are retroperitoneal, whereas the transverse colon and sigmoid are intraperitoneal (Figure 30–1). The diameter of the lumen is greatest at the cecum (~ 7 cm), and decreases distally. As a result, mass lesions of the cecum are least likely to cause obstruction and the thin wall of the cecum is most vulnerable to ischemic necrosis and perforation from large bowel obstructions. There are four layers of the wall—mucosa, submucosa, muscularis propria, and serosa (Figure 30–2). The mucosa is composed of three layers—a simple columnar epithelium organized to form crypts, lamina propria, and muscularis mucosa. The submucosa is the strength layer of the colon because it has the highest concentration of collagen. Therefore, this layer is especially important to incorporate during anastomoses. The muscularis propria is composed of an inner circular layer and an outer longitudinal layer that thickens into three bands around the circumference to form the taeniae coli. The appendix can be found at the point on the cecum where the taeniae converge. At the rectosigmoid, these bands fan out to form a uniform layer, marking the end of the colon and the beginning of the rectum. The forces of these muscular components of the wall result in shortening of the colon to form sacculations called haustra (Figure 30–3). These are not fixed structures, but can be observed to move longitudinally. The appendices epiploicae are fatty appendages on the serosal surface.
The rectum begins at the sacral promontory and ends at the anorectal ring. It lies between the colon and the anus and is 12-16 cm in length. There are no taeniae as the longitudinal muscle fans out and encompasses the circumference of the rectal wall. The rectum can be further differentiated from the colon by its lack of appendices epiploicae and haustra. The rectum is both an intra- and extraperitoneal organ. Posteriorly, the rectum is only intraperitoneal at the level of the rectosigmoid. However, the anterior and lateral walls of the upper rectum are intraperitoneal. The anterior peritoneal reflection extends low into the pelvis, approximately 5-8 cm above the anal verge and lies between the rectum and the bladder in men and uterus in women (the Pouch of Douglas). Tumors or abscesses in this location can be palpated on digital rectal or vaginal examination. Denonvilliers fascia envelops the anterior wall of the rectum. Beyond the anterior peritoneal reflection lie the seminal vesicles and prostate gland in men. In women, the cervix and rectovaginal septum lie anteriorly and the adnexa anterolaterally. The rectum has three major mucosal folds called the valves of Houston. They are variable in location, but classically occur every 3-4 cm. The superior and inferior folds are on the left and the middle fold is on the right. The middle fold marks the distal extent of the intraperitoneal rectum.
The pelvic floor is made of three muscles—the pubococcygeus, iliococcygeus, and puborectalis collectively known as the levator ani. The puborectalis forms a sling at the anorectal junction and is an important component of continence and the defecation mechanism.
The arterial supply of the colon is dictated by its embryological origin. The foregut, which extends to the distal transverse colon, is supplied by the superior mesenteric artery (SMA) through the ileocolic, right colic, and middle colic arteries. The hindgut, which includes the distal transverse colon to the rectum, is supplied by the inferior mesenteric artery (IMA). This gives off the left colic, 2-6 sigmoid branches, and terminates as the superior rectal (hemorrhoidal) artery. The middle and inferior rectal (hemorrhoidal) arteries originate from the internal iliac artery. There is redundancy in the arterial circulation via the meandering mesenteric artery (Arc of Riolan) as well as the marginal artery of Drummond, which is a network of arterial branches that runs the length of the colon approximately 2.5 cm from the bowel wall. The vasa recta are the terminal arteries arising from the marginal artery. These penetrate the colon wall in multiple areas on the mesenteric side, creating focal areas of weakness that have the potential to form false diverticula. The left colic, sigmoid, and superior rectal branches also originate from the IMA in a variable pattern. The venous drainage generally follows the arterial supply with the exception of the inferior mesenteric vein, which runs lateral to the ligament of Treitz to join the splenic vein. The vascular supply too is highly variable, and “normal” anatomy is found in only 15% of individuals.
The lymphatics drain via continuous plexuses in the submucosal and subserosal layers of the bowel wall. These continue on to the mesenteric lymphatic channels and nodes that accompany the blood vessels, which explain why standard planning for oncologic resections of the colon and rectum is based on the vascular supply.
The nerve supply to the colon and rectum is autonomic and, like the blood supply, is divided according to embryologic development. Sympathetic nerves to the midgut, including the right and proximal transverse colon, originate in T6-12. These synapse in the superior mesenteric plexus and then follow the SMA and its branches to the right colon. The vagus nerve provides parasympathetic fibers to the midgut. Sympathetic innervation to the hindgut (distal transverse colon to the rectum) arises from L1-3. These sympathetic fibers synapse in the preaortic plexus and then follow the IMA to the bowel wall. Nervous impulses to the bowel wall synapse in the myenteric (Auerbach’s) plexus and submucosal (Meissner’s) plexus. The myenteric plexus is located in between the circular and longitudinal layers of smooth muscle and is responsible for coordinating motility. The submucosal plexus (Meissner’s plexus) is located in the submucosa and regulates secretions, blood flow, and absorption.
The distal rectum and anus is innervated by the hypogastric nerves and the nervi erigentes. The hypogastric nerves are sympathetic fibers that originate from L1-3. These fibers course over the sacral promontory to the hypogastric plexus and form the paired hypogastric nerves near the root of the IMA. The parasympathetic fibers run in the nervi erigentes, which arise from S2-4 and join the hypogastric nerves near the lateral rectal stalks. The fibers continue anterior and laterally to innervate the rectum, pelvic floor muscles, and bladder, as well as the prostate and seminal vesicles in men. The location of these nerves makes them prone to injury during protectomy. The superior hypogastric plexus is at risk during high ligation of the IMA, the hypogastric nerves are at risk during retrorectal dissection, the inferior hypogastric plexus and nervi erigentes are at risk during mobilization of the lateral stalks, and the periprostatic plexus is at risk during dissection of Denonvillier fascia. Injury to these nerves during proctectomy can cause bladder and sexual dysfunction that is a significant source of postoperative morbidity.
PHYSIOLOGY
The primary functions of the colon are absorption, secretion, motility, and intraluminal digestion. These interrelated processes are responsible for converting ileal effluent into semisolid feces. The rectum functions as a capacitance organ, storing feces produced by the colon and allowing defecation at a convenient time. When surgery or disease results in loss of colonic function, there is a significant increase in intestinal losses of water and electrolytes. If the rectum is overloaded by large volume watery stool or its capacity to distend is lost or impaired by surgery or disease, fecal urgency and frequency are noted.
Normal volume and composition of intestinal gas has great variation amongst individuals depending on air swallowing, diet, and the composition of the microbial flora. Small amounts of intestinal gas are absorbed through the bowel wall and excreted through the lungs, but the majority (~ 400-1200 mL/d) is discharged as flatus.
Intestinal gas is comprised of nitrogen, oxygen, carbon dioxide, hydrogen, methane, and trace substances such as methyl sulfide, hydrogen sulfide, indole, and skatole. By far, the largest component is nitrogen, which makes up 30%-90% of intestinal gas. Most nitrogen comes from swallowed air, but some also diffuses from the plasma. Fermentation of nonabsorbed carbohydrates such as fiber is responsible for most of the hydrogen and carbon dioxide in gas. In lactase deficient individuals, lactose fermentation contributes as well. Methane is produced by specific hydrogen reducing bacteria (Methanobrevibacter smithii) and is only present in 30% of people. Both methane and hydrogen gases are explosive and require caution when using cautery in the bowel lumen.
Patients reporting crampy abdominal pain, bloating, and increased flatus may be suffering from increased gas production. Overproduction may be a result of a malabsorptive state and eliminating lactose, legumes, and/or wheat in the diet may be of help.
The colon is primarily an absorptive organ. The absorptive capacity is greatest in the cecum and ascending colon and decreases distally. Most nutrients are absorbed in the small bowel; however, when enteric contents reach the colon, they are still rich in water, electrolytes, and some nutrients. Approximately 1-2 L of ileal effluent containing 250 mEq of sodium reaches the cecum every 24 hours. The passive absorption of water and active transportation of sodium allows recovery of more than 90% of the water and sodium content. Every 24 hours, approximately 100-200 mL of water and 2-5 mEq of sodium are excreted in the feces. Table 30–1 gives average values for the composition of ileal effluent and feces. Normal feces are composed of 70% water and 30% solids. Bacteria constitute over half of the solid component. The remaining solids are food waste and desquamated epithelium. Although the absorptive capacity of the colon serves an important role in maintaining homeostasis, it is not essential to life.
| Ileal Effluent | Feces | Net Colonic Absorption (per 24 hours) | ||||
|---|---|---|---|---|---|---|
| Concentration (mEq/L) | Quantity (per 24 hours) | Concentration (mEq/L) | Quantity (per 24 hours) | Normal | Maximal Capacity | |
| Na+ | 120 | 180 mEq | 30 | 2 mEq | +178 mEq | +400 mEq |
| K+ | 6 | 10 mEq | 67 | 5 mEq | −5 mEq | ±45 mEq |
| CI− | 67 | 100 mEq | 20 | 1.5 mEq | +98 mEq | +500 mEq |
| HCO3 | 40 | 60 mEq | 50 | 4 mEq | +56 mEq | |
| H2O | 1500 mL | 100 mL | +1400 mL | +5000 mL | ||
This effluent also contains small amounts of nutrients which are absorbed by the colon including fatty acids, amino acids, and vitamin K. Approximately 10% of undigested starch reaches the colon where colonic bacteria fermentation produces short chain fatty acids, which are important both systemically and as a nutrient source for colonocytes.
In addition, the colon is part of the enterohepatic circulation. Bile acids not absorbed in the terminal ileum are passively absorbed in the colon. When this capacity is exceeded, the remaining bile acids are metabolized by colonic bacteria into urobilin and stercobilin. Urobilin, stercobilin, and their metabolites are responsible for giving stool its brown color.
The colon also serves an important secretory function. It secretes hydrogen, bicarbonate, chloride, and potassium ions. The colon is in communication with the circulation and is able to adjust over a wide range as needed. Pathologic states such as inflammatory bowel disease (IBD), shigellosis, cystic fibrosis, and collagenous colitis cause electrolyte and acid-base disturbances by altering colonic secretion.
Colonic motility serves to maximize absorption and move feces distally in preparation for excretion. There are numerous types of motility and these occur with great variation along the length of the colon. Normal movements are slow, variable, and complex and result in nonorganized flow of the fecal stream. In patients with normal bowel function, enteric contents reach the cecum 4 hours after a meal and the rectosigmoid by 24 hours. However, portions of the fecal stream are mixed so that new effluent may bypass old effluent, and residue from a single meal may pass in bowel movements over 3-4 days.
The enteric nervous system coordinates motility. It is increased by physical activity, stress, and diets high in fiber. In addition, the act of eating stimulates colonic transit. The gastrocolic reflex refers to the activation of colonic motor activity in response to a meal. The magnitude is determined by the fat and caloric composition. This increased activity increases ileal and colonic emptying, causing an urge to defecate.
The colon has three distinct patterns of activity that are under control of the enteric nervous system and a postulated pacemaker in the transverse colon. Retrograde peristalsis consists of annular contractions occurring most commonly in the right colon. These contractions work to keep the stool in the right colon and therefore facilitate absorption. As ileal effluent continues to empty into the cecum, some of the liquid stool flows to the transverse and descending colon, where segmentation predominates. This action consists of random, uncoordinated annular contractions over short segments that propel feces both proximally and distally. Finally, mass movement occurs as a strong coordinated contraction that starts proximally and propels colonic contents distally and occurs in concert with the gastrocolic reflex.
There is a broad range of normal bowel habits that varies across cultures. A cross-sectional study of 20,000 men and women across all age groups in Britain showed an average of 1-1.5 bowel movements per day, which increased with fiber and fluid intake and physical activity.
The urge to defecate is stimulated by rectal stretch in response to feces. This elicits the rectoanal inhibitory reflex which causes relaxation of the internal anal sphincter and allows the rectal contents to be “sampled” by specialized mucosa in the anorectal transition zone. This mucosa contains sensory fibers that can discriminate between flatus and solid and liquid stool. Immediately following this sensory process, the external sphincter contracts and the contents are moved proximally back into the rectum. Flatus and stool can be discharged, if appropriate. If not, the rectum relaxes and the urge to defecate passes. Normal defecation requires regular colonic function, specifically with regards to stool consistency, in addition to coordinated colonic motility, rectal sensation, sphincter function, and pelvic floor relaxation. When the rectum senses presence of feces and the decision is made to defecate, intra-abdominal pressure is increased and the pelvic floor and anal sphincters relax, allowing the rectum to straighten. After elimination of the stool bolus, the sphincters and pelvic floor resume their tone.
Diarrhea is defined by the loss of more than 300 mL of fluid per day and chronically affects 5% of the United States population. It can result in severe electrolyte disturbances and dehydration. There are four main etiologies of diarrhea—invasive, secretory, osmotic, and malabsorptive. Invasive diarrhea results when enterocytes are destroyed by pathogens such as Shigella, Campylobacter, and Entamoeba histolytica, resulting in low volume loose bowel movements with blood and leukocytes. Secretory diarrhea is the result of excessive secretion by colonocytes or enterocytes. This results in high volume, isotonic diarrhea with a low osmotic gap. The primary causes are enterotoxins produced by E coli and Vibrio cholera, and excessive serotonin secretion in the carcinoid syndrome. Osmotic diarrhea occurs when an osmotically active substance in the bowel lumen draws hypotonic fluid from the circulation. Osmotic diarrhea is characterized by high volume output with a high osmotic gap and no leukocytes. Most commonly, this occurs in patients with disaccharidase deficiencies such as lactose intolerance. Malabsorptive diarrhea results from the lack of normal digestive or absorptive processes. Pancreatic insufficiency results in loss of proteolytic and lipase activity causing steatorrhea. Whipple’s disease (caused by infection with Tropheryma whippelii) and celiac sprue (autoantibodies to gluten) are characterized by inflammatory changes with loss of absorptive capacity of the small bowel. Surgery can also cause malaborption. Extensive small bowel or colonic resections compromise the absorptive capacity and may result in diarrhea. A portion of patients undergoing cholecystectomy will also experience transient diarrhea with fatty meals due to the loss of the bile reservoir capacity of the gallbladder and subsequent steatorrhea.
CONSTIPATION
Based on an epidemiologic review of the literature, constipation affects 7%-79% of the United States population with a mean of 16%. The wide variation in reports is likely due to broad definitions of constipation including infrequent or hard stools, excessive straining, or incomplete evacuation. Incidence is associated with lower socioeconomic and educational status, non-Caucasian ethnicity, female gender, and increasing age. Low fiber intake is especially important, as it normally bulks stool, triggering colonic motility.
Although idiopathic constipation occurs, changes in bowel habits should prompt a search for a cause. Numerous medications cause constipation, such as opiates, antiemetics (ondansetron), antipsychotics (chlorpromazine, haloperidol, risperidone, clozapine), antihypertensives (calcium channel blockers, atenolol, furosemide, clonidine), as well as over the counter medications such as ibuprofen, calcium, and iron supplements. The patient should be asked about previous laxative use, as rebound constipation may occur in patients with a history of laxative abuse. Constipation can be a symptom of a systemic disease such as hypothyroidism, hyperparathyroidism, diabetes, electrolyte disturbance, or connective tissue disorders. It may result from a primary colonic disorder such as Hirschsprung, endometriosis, and benign or malignant strictures. Neurologic disease or injury, psychiatric illness, and physical or sexual abuse may be contributory.
If none of these factors is present, patients may be suffering from a functional constipation syndrome, such as colonic inertia, irritable bowel syndrome, or pelvic floor dysfunction. Patients with colonic inertia report lifelong constipation with laxative dependence. Irritable bowel syndrome (constipation subtype) causes irregular bowel habits, bloating, and abdominal pain classically relieved with bowel movements. Patients with pelvic floor dysfunction or obstructed defecation syndrome report incomplete evacuation and excessive straining that is often improved with digital manipulation. They may have coexistent urinary dysfunction, pelvic organ prolapse, and sexual dysfunction.
Evaluation for constipation includes a history and physical examination, including review of medications, and digital rectal and anoscopic evaluation. Metabolic etiologies may be ruled out with laboratory tests including TSH, calcium, electrolytes, and blood sugar in the appropriate patient. Anatomic lesions can be identified by colonoscopy. This should be considered in any patient with alarm symptoms (hematochezia, anemia, or weight loss), refractory constipation, and patients who are due for age-appropriate colon cancer screening. Medical therapy with fiber supplementation and laxatives should be tried first. Patients should be encouraged to keep a written record of their bowel habits. In refractory cases, and those in which a functional disorder is suspected, additional testing is indicated.
Anorectal manometry and balloon expulsion test should be considered in severe, refractory cases of constipation where the etiology is not clear and in patients with symptoms of pelvic floor dysfunction. Manometry allows evaluation of the rectoanal inhibitory reflex, sphincter tone, rectal sensation, and coordination. Patients with constipation tend to exhibit a hypertonic internal sphincter with poor squeeze pressures. Electromyography may reveal nonrelaxation or paradoxical contraction of the puborectalis. Balloon expulsion can be performed as an adjunctive study. It involves placing a balloon in the rectum and filling it until the urge to defecate is perceived. The patient is then asked to evacuate the balloon. Normal function is indicated by the ability to evacuate a 50-100 mL balloon in less than 1 minute. This simple test was shown to have 88% sensitivity and 89% specificity for diagnosing defecatory disorders compared with defecography. Defecography (barium, scintigraphic, or magnetic resonance) is useful when the results of manometric testing are not diagnostic.
Colonic transit can be evaluated using radiopaque marker studies. Patients ingest 24 markers and avoid laxatives. An abdominal radiograph is taken on the fifth day with note made of the distribution and number of retained markers. Normal patients will have fewer than five retained markers. In fact, eighty percent of normal patients will have passed all markers by the fifth day. Colonic inertia, a primary disorder of colonic motility, is diagnosed when more than five markers are retained and are scattered throughout the colon. Obstructed defecation is suggested if markers have accumulated in the rectum. A more detailed examination involves ingestion of radiopaque markers daily for three consecutive days and taking abdominal films on the fourth and seventh days. The number and distribution of retained markers are compared with established normal controls.
Initial treatment of constipation should focus on lifestyle and dietary modification. Physical activity, fluid intake, and consumption of fruits, vegetables, and whole grains are increased. A fiber supplement is started in an effort to soften and bulk the stool. A low dose is recommended at first and slowly increased to the desired effect to minimize bloating and flatulence.
Laxatives, stimulants, and enemas should be used only for short periods of time for treatment of acute discomfort. Osmotic laxatives include lactulose, magnesium hydroxide, sodium phosphate, and polyethylene glycol (MiraLAX). These work by increasing the intraluminal osmolarity, creating a gradient that reduces fluid reabsorption. Magnesium hydroxide should be avoided in patients with renal insufficiency. Polyethylene glycol is one of the most commonly recommended laxatives as it is safe and effective, even for use in the long term. Colonic irritants work by increasing motility. Examples include senna, cascara, and bisacodyl. These medications can be limited by crampy abdominal pain. Long-term use of senna and cascara causes melanosis coli (brown discoloration of the mucosa). Stool softeners such as mineral oil and docusate work by increasing stool fluid retention. Enemas stimulate bowel movements by causing direct rectal stretch and irrigation (saline, soap suds), or softening of the stool (mineral oil). Colonic irritants and enemas can cause reduced colon and rectal motility and should not be used for prolonged periods of time. Lubiprostone, linaclotide, and prucalopride are newer agents indicated for the treatment of refractory idiopathic constipation and constipation-predominant irritable bowel syndrome. They cause increased secretion via activation of chloride channels (lubiprostome and linaclotide) and 5-HT4 receptors (prucalopride).
Defecatory disorders, as diagnosed by anorectal manometry, are best managed using biofeedback and pelvic floor retraining. Patients can learn to relax and strengthen the pelvic floor muscles and can develop increased rectal sensation awareness. These techniques have been studied in a randomized fashion and have been shown to be superior to medical management.
Selected patients with constipation benefit from surgery if all functional aspects of defecation have already been addressed. If there is pelvic floor dysfunction, this should be first addressed using biofeedback. Surgery is considered if patients have obstructed defecation from mechanical reasons such as rectal prolapse, rectocele, enterocele, or sigmoidocele may benefit from procedures to address the specific condition. Patients with colonic inertia without pelvic floor dysfunction, irritable bowel syndrome, or diffuse upper gastrointestinal involvement are treated with abdominal colectomy with ileorectal anastomosis. Multiple other approaches, including segmental colectomy and preservation of the cecum have shown conflicting, but overall worse, outcomes. Complications have been reduced with the use of laparoscopic techniques, which have been shown to be safe and effective. The antegrade colonic enema procedure is used more often in pediatric patients. It involves bringing up a piece of bowel (appendix, ileum, cecum, and left colon have been described) to the abdominal wall to allow intermittent catheterization with irrigation of the colon. This technique has been reported in adults with obstructive defecation and neurologic deficits with success. Newer therapies such as sacral nerve stimulation are currently under investigation.
MICROBIOLOGY
The colon of the fetus is sterile but the process of colonization begins immediately after birth. There is marked variation in the composition of the microbial flora between individuals, likely related to genetic, environmental, and dietary factors. Humans have 10 times more bacterial cells in their bodies than human cells, and in fact, bacteria make up 50% of the dry weight of feces. Microbes are present throughout the gastrointestinal tract, and their concentration increases from proximal to distal, with the highest concentrations in the colon and rectum. The majority of colonic organisms are anaerobic, but there is a substantial population of facultative anaerobes. Bacteroides spp account for the 60%-70% of the bacterial flora. Other predominant organisms include Escherichia coli, Lactobacillus bifidus, Kelbsiella, Proteus, Clostridium, Enterobacter, and Enterococcus. Methanobrevibacter smithii is responsible for methane production.
These bacterial populations are instrumental for the health of the colon. They serve a barrier function and maintain epithelial integrity. Fermentation of undigested polysaccharides creates short chain fatty acids that are an important energy source for colonocytes. In addition, bacteria play an active role in the immune function of the gut. Healthy bacterial populations make it difficult for pathogens to establish infection. In addition, normal microbial flora also serves a role in numerous physiologic processes. They deconjugate unabsorbed bile acids, creating the pigments that give stool its characteristic color, synthesize vitamin K for systemic absorption and use, and recycle colonic nitrogen in the form of urea.
DISEASES OF THE COLON AND RECTUM
Fifteen percent of intestinal obstructions in adults occur in the large bowel and the incidence increases with age. Obstruction can result from actual pathology of the bowel wall including malignancy and strictures, mechanical problems such as volvulus, incarcerated hernia, and intussusception, or intraluminal factors such as fecal or foreign body impaction (Table 30–2). Benign strictures are commonly associated with diverticulitis or IBD, but may occur as a result of ischemia, radiation, or as a result of a surgical anastomosis. Acute functional obstruction of the colon (Ogilvie syndrome) can cause the same spectrum of clinical symptoms. It is important to differentiate pseudo-obstruction from mechanical obstruction because the management is different. In contrast to small bowel obstruction, adhesive disease of the large intestine is a very rare cause.
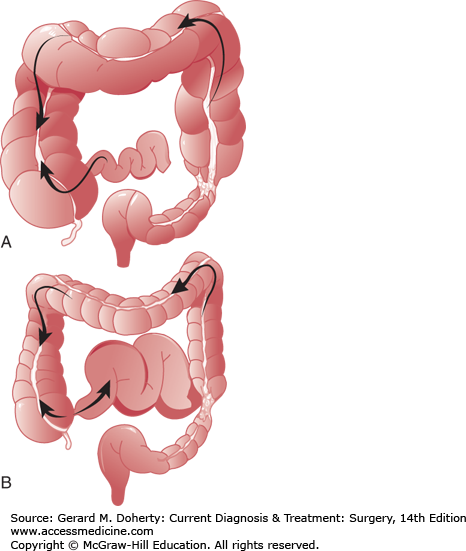
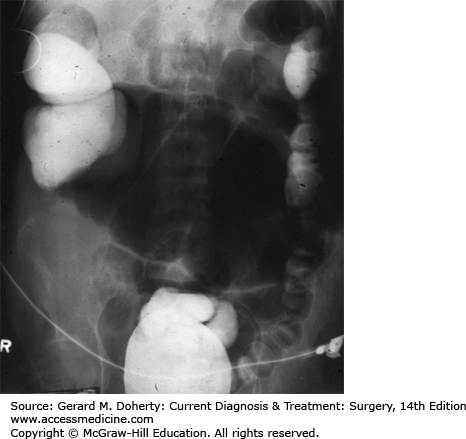
A major factor in the clinical course of large bowel obstruction depends on the competence of the ileocecal valve. Ten to twenty percent of patients have an incompetent ileocecal valve which allows decompression of the large bowel contents into the ileum. However, in the majority of patients, the ileocecal valve does not allow reflux to occur, resulting in a closed loop obstruction with rapidly increasing intraluminal pressure (Figure 30–4). This results in impaired capillary circulation, mucosal ischemia, and subsequent bacterial translocation with systemic toxicity. This process ultimately progresses to gangrene and perforation. This same process occurs in volvulus, which by definition is a closed loop obstruction. The cecum has the largest diameter and therefore, by the law of LaPlace, is at greatest risk for perforation. The normal diameter is approximately 7 cm. The risk of perforation is high if the diameter increases acutely or to a size greater than 10-12 cm.
Figure 30–4.
The role of the ileocecal valve in obstruction of the colon. The obstruction is in the upper sigmoid. A: The ileocecal valve is competent, creating a closed loop between the obstruction and the valve. Tension in the closed loop is increased further by emptying of gas and fluid from the ileum into the colon. B: The ileocecal valve is incompetent. Reflux into the ileum is permitted. The colon is relieved of some of its distention, and the small bowel has become distended.
The history and physical examination can help distinguish a large bowel obstruction from other causes of an acute abdomen and identify those patients requiring urgent therapy. Obstipation is a universal feature of complete obstruction, though the patient may pass stool and gas located distal to the obstruction after the initial symptoms begin. Classically, the digital rectal examination reveals an empty vault except in cases of distal fecal impaction. Vomiting is a late finding and may not occur at all if the ileocecal valve prevents reflux. If reflux decompresses the cecal contents into the small intestine, the patient may also present with symptoms of small bowel obstruction.
The onset of symptoms maybe acute or gradual, depending on the location and etiology of the obstruction. A patient may report a history of constipation for months preceding any acute obstructive symptoms. Deep, visceral, cramping pain from obstruction of the colon is usually referred to the hypogastrium. Right sided lesions tend to grow to a large size prior to causing obstruction owing to the larger diameter of the lumen and the liquid stool. Therefore, these lesions may be palpable on abdominal examination. If the patient reports repeated episodes of fever and abdominal pain, a diverticular stricture is suspected; a history of hematochezia and weight loss suggests colorectal cancer (CRC). Alternatively, when symptoms occur acutely, volvulus, incarcerated hernia, or intussusception are more likely. A patient with fever, leukocytosis, and peritonitis has likely progressed to develop intestinal ischemia and/or perforation.
Abdominal films will frequently reveal dilated colon outlining the abdominal cavity. The colon can be distinguished from the small intestine by its haustral markings, which do not cross the entire lumen of the distended colon. Sigmoid volvulus can be identified by a characteristic “coffee bean” appearance which represents a dilated loop of colon starting in the left-lower quadrant extending medially. This finding is seen in 60% of patients. Cecal volvulus tends to appear as a dilated loop originating in the right-lower quadrant extending medially. In patients with other forms of obstruction, the extent of distention is dependent on the competency of the ileocecal valve and its relation to the location of the obstruction. A transition point with no distal colonic gas indicates complete obstruction. If gas is present in the distal rectum, it can represent residual material present prior to the obstruction or the presence of a partial obstruction. In acute pseudo-obstruction, the colon is diffusely dilated with stool and gas throughout (Figure 30–5). A CT scan with rectal contrast is the most useful single test for large bowel obstruction because it can yield information regarding the location and etiology of the bowel obstruction. This has largely replaced contrast enema. A water-soluble contrast medium, such as gastrografin, should be used if strangulation or perforation is suspected. Barium should not be given orally in the presence of suspected colonic obstruction.
An operation is almost always required for mechanical large bowel obstructions. The extent of surgery depends on the patient’s acuity and the etiology of the obstruction. The primary goals of treatment are resection of all necrotic bowels and decompression of the obstructed segment. Removal of the obstructing lesion is a secondary goal, but a single operation to accomplish both objectives is preferred whenever possible. Options include resection with primary anastomosis, resection with diversion, diversion alone, and endoscopic stent placement.
Endoscopic stents are being used as a bridge to surgery and as palliative therapy. When used in acutely ill patients with a malignancy, there is no clear advantage of endoscopic stent placement as a bridge to surgery in terms of mortality or complications. However, they do allow decompression of the obstruction and time for physiologic optimization. This can increase the chances of resection with primary anastomosis and reduce the chance for diversion. This comes, however, at the risk of stent-related perforation. Stents may be considered also for palliation in high risk patients whose obstructing cancers are not resectable; however, if the patient will tolerate it, a permanent diverting colostomy is a more durable option.
Generally speaking, obstructing lesions of the right colon can be resected in one stage if the patient’s condition is stable. If the patient’s condition is precarious or if the colon has perforated, the bowel should be resected and an ileostomy created. Intestinal continuity can be restored at a second operation. Intestinal bypass is sometimes used for unresectable lesions although the relief of the obstruction is not always as effective.
Obstructing lesions of the left colon more commonly require diversion. Ideally the lesion is resected at the initial operation. After resection, anastomosis may be postponed with creation of a temporary end colostomy and Hartmann’s pouch (Figure 30–6). If the patient’s clinical condition will allow it, a primary anastomosis may be performed with a diverting loop ileostomy if needed to protect the anastomosis. If resection is not possible, the bowel can be decompressed proximally and distally with a loop or double barrel colostomy. These stomas are difficult to manage and are associated with a high rate of prolapse and are thus, avoided if at all possible.
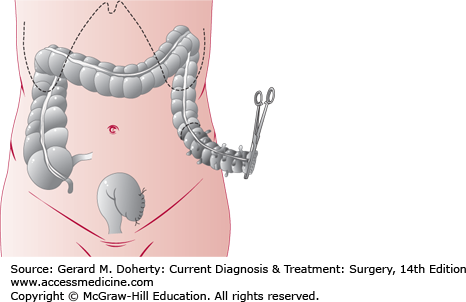
Figure 30–6.
Primary resection for diverticulitis of the colon, also used in acute large bowel obstructions. The affected segment (shaded) has been divided at its distal end. If primary anastomosis is to be done, the proximal margin (dotted line) is transected, and the bowel is anastomosed end to end. If a two-stage procedure will be used, a colostomy is formed at the proximal margin, and the distal stump is oversewn (Hartmann procedure, as shown) or exteriorized as a mucous fistula. The second stage consists of colostomy takedown and anastomosis.
The prognosis depends upon the age and general condition of the patient, the extent of vascular impairment of the bowel, the presence or absence of perforation, the cause of obstruction, and the promptness of surgical management. The overall mortality rate is about 20%. Cecal perforation carries a 40% mortality rate. Obstructing cancer of the colon has a worse prognosis than nonobstructing cancer because it is more likely to be locally invasive or metastatic to nodes or distant sites.
Acute pseudo-obstruction of the colon (Ogilvie syndrome) presents with massive colonic distention in the absence of a mechanically obstructing lesion (Figure 30–5). It is a severe form of ileus that occurs most commonly in systemically ill patients and results from an imbalance in the autonomic tone with subsequent absence of peristalsis. Electrolyte disturbances and medications are contributing factors. These patients are usually recovering from major surgery or are hospitalized for other causes, most commonly cardiac disease, trauma, and infection. The mortality rate is 15% overall but increases to 30% in patients who develop ischemia or perforation.
The initial presentation may be missed in critically ill patients. Abdominal distention is the earliest manifestation, but later symptoms include abdominal pain, vomiting, and obstipation and may mimic those of true obstruction; however, 40% of patients will have diarrhea. The differential diagnosis includes toxic megacolon (in patients with ulcerative colitis [UC] and C difficile colitis) and mechanical large bowel obstruction. Plain x-rays of the abdomen show marked distention of the colon, often most severe in the right and transverse colon. Contrast enema proves the absence of obstruction, but instillation of radiopaque material should cease as soon as the dilated colon is reached. As opposed to a mechanical bowel obstruction, surgical management in Ogilvie syndrome is reserved for complications including perforation and ischemia.
If the patient has no signs of obstruction or perforation, the initial measures include nasogastric suction, rectal tube placement, fluid resuscitation, and correction of electrolyte imbalances. Systemic illnesses contribute and should be treated appropriately (respiratory failure, cardiac disease, and sepsis). All anticholinergic and narcotic medications should be discontinued. These measures will be effective in 75%-85% of patients. The patient should be followed with serial imaging. The risk of perforation is related to cecal diameter. The highest risk patients have a diameter > 10 cm and should be closely monitored. If there is any sign of clinical deterioration or no improvement in 48 hours, more aggressive treatment is warranted.
The acetylcholinesterase inhibitor neostigmine is an effective treatment for acute colonic pseudo-obstruction in patients without response to conservative measures. It works by acutely increasing acetylcholine levels in the body thereby prompting immediate bowel contraction and decompression. Patients require telemetry monitoring during and after administration as symptomatic bradycardia requiring atropine occurs in 10% of patients. The most common side effects include abdominal cramping and excessive salivation. It is successful in 90% of patients with a recurrence rate of 7%. If this is unsuccessful, or neostigamine is contraindicated (renal insufficiency, pregnancy, bronchospasm, bradycardia), colonoscopic decompression is the next step in management. Endoscopic decompression carries a risk of perforation but when performed by an experienced team, the risk of perforation is reduced to 2%. This procedure also offers the benefit of visualization of the colonic mucosa for evidence of ischemia. This is initially successful in 80% of patients, but recurrence is common. Ideally a tube is placed into the right colon using fluoroscopic guidance to maintain decompression.
In patients with refractory disease, surgical intervention is required. A percutaneous cecostomy tube is possible in patients who are high risk for surgery. If there is evidence of ischemia or perforation, patients are offered segmental or subtotal colectomy depending on the distribution of disease. This operation carries a high morbidity and mortality, likely related to patient’s underlying illness. Primary anastomosis is considered based on the patient’s clinical status, but often diversion is performed and intestinal continuity created at during a second procedure months after resolution of disease.
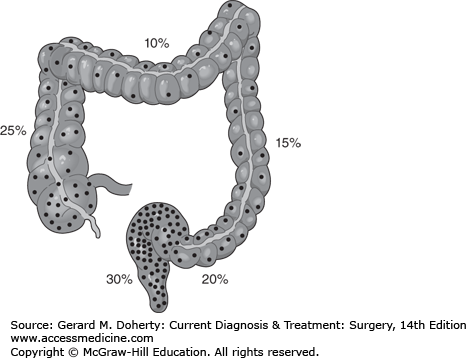
In the United States, colorectal cancer (CRC) ranks third after prostate and lung cancer in men, and after breast and lung cancer in women in both incidence and mortality. The American Cancer Society predicts that in 2012, more than 143,000 patients will be diagnosed and 51,000 will die as a result of their disease. Men have a higher incidence and mortality than women, and Black Americans have a higher incidence and mortality than other ethnic groups. The overall incidence and mortality from CRC has been decreasing since the early 1980s, likely owing to improved screening. The frequency of CRCs by location in the colon is shown in Figure 30–7. The average age at diagnosis is 68 years for men and 72 years for women.
The average lifetime incidence of CRC in the United States is more than 5%. Multiple risk factors for CRC have been identified. The most significant are considered non-modifiable. For instance, having a single first degree family member with a diagnosis of CRC increases one’s lifetime risk by 2.2. If the relative is diagnosed at an early age, the risk is increased by 3.9 and if there is more than one relative, this risk increases to 4.0. The most significant personal risk factors include IBD, especially with pancolitis, a history of adenomatous polyps, diabetes, and obesity. Modifiable risk factors include increased red and processed meat consumption, smoking, and alcohol. Case control and cohort studies have suggested that protective factors include a high fiber diet, calcium as well as vitamin D supplementation and physical activity. Randomized controlled intervention trials have been unable to demonstrate that these interventions significantly reduce risk for CRCs; however, they are limited by the older age of the study population at the time of intervention, poor adherence, and a relatively short follow up. A large Danish cohort study concluded that 25% of cancers are preventable through a healthy lifestyle.
In addition to lifestyle, chemoprevention in colon cancer has also been suggested as a way to reduce the burden of disease. Celecoxib and low dose aspirin have been shown to reduce the rate of metachronous adenomas in average risk patients found to have adenomas on screening colonoscopy. However, the adverse effects of these medications including cardiovascular events with celecoxib and peptic ulcer disease with aspirin likely outweigh these risks and therefore their use is limited in average risk patients. However, the benefits of celecoxib are more likely to outweigh the risk in patients with familial adenomatous polyposis (FAP), an inherited CRC syndrome, and it has been FDA approved for this indication.
CRC develops through a stepwise accumulation of mutations that allow the progression of normal mucosa to adenoma to carcinoma in a pathway known as Loss of Heterozygosity. The inciting event in 85% of sporadic CRCs is the development of chromosomal instability. This allows a cell to accumulate inactivating mutations in tumor suppressors such as adenomatous polyposis coli (APC), P53, deleted in colorectal carcinoma (DCC) and SMAD 4, as well as activating mutations in oncogenes such as K-ras, c-myc, c-src, and BRAF in a stepwise fashion. A less common pathway involves the development of mutations in the genes responsible for DNA mismatch repair and subsequent microsattelite instability. A third mechanism involves the epigenetic silencing of tumor suppressor genes though abnormal methylation. The familial cancer syndromes are caused by germline mutations in these genes, such as APC in familial adenomatous polyposis and mismatch repair genes in Lynch Syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC). Approximately 5% of CRCs occur in patients with a hereditary syndrome.
The most common genetic colon cancer syndrome is Lynch syndrome, formerly known as HNPCC. The name of the condition has reverted to Lynch syndrome as these patients may have polyps in addition to cancer. It is an autosomal dominant condition with 80% penetrance whose basis is a mutation in the DNA mismatch repair system clustered on chromosome 2p. Ninety percent of all patients have a mutation in MLH1 or MSH2 which results in microsatellite instability (MSI). Other known mutations include MSH6, PMS2, and PMS1. The lifetime risk of CRC is 66% in men and 43% in women with a median age at diagnosis of 42 and 47 years, but this varies between affected families depending on the mutation. These cancers tend to occur in the ascending colon. A hallmark of the disease is the association with other cancers, including the endometrial, ovarian, gastric, upper urinary tract, biliary, small bowel, and brain.
The diagnosis is usually suspected on clinical grounds using the patient’s medical and family history. Four sets of criteria were developed to help make the diagnosis. The Amsterdam criteria were initially developed in 1990 and are based on an accurate, extended family history of colon cancer. They were found to have a sensitivity of 61% and a specificity of 67% for the diagnosis. In 1999 the Amsterdam II criteria were published, which increased the sensitivity by including extracolonic cancers (Table 30–3a). Patients who meet either of these criteria should be offered genetic testing, which can be used to provide diagnostic and prognostic information for the patient and his or her family. A separate set of criteria, known as the Bethesda Guidelines, was published in 1997 (Table 30–3b). These were designed to have very high sensitivity (94%) at the expense of specificity (49%) and are used to select tumor specimens appropriate for MSI testing. If this test is positive, it is followed by a confirmatory genetic test for the HNPCC mutations, because 15% of sporadic CRC exhibit MSI.
| Amsterdam I Criteria | |
| At least three relatives must have histologically verified colorectal cancer. | (1) One must be a first-degree relative of the other two. |
| (2) At least two successive generations must be affected. | |
| (3) At least one of the relatives with colorectal cancer must have received the diagnosis before age 50. | |
| Amsterdam II Criteria | |
| At least three relatives must have a cancer associated with hereditary nonpolyposis colorectal cancer (HNPCC) (colorectal, endometrial, stomach, ovary, ureter or renal-pelvis, brain, small bowel, hepatobiliary tract, skin [sebaceous tumors]) | (1) One must be a first-degree relative of the other two. |
| (2) At least two successive generations must be affected. | |
| (3) At least one of the relatives with HNPCC-associated cancer must have received the diagnosis before age 50. |
| 1. Colorectal cancer (CRC) diagnosed in individual under age 50 years. |
| 2. Presence of synchronous, metachronous colorectal or other HNPCC-associated tumors, regardless of age. |
| 3. CRC with the microsatellite instability-high (MSI-H) histology (presence of tumor-infiltrating lymphocytes, Crohn-like lymphocytic reaction, mucinous/signet-ring differentiation, or medullary growth pattern) in patient 60 years of age. |
| 4. CRC in one or more first-degree relatives with an HNPCC-related tumor, with one of the cancers being diagnosed under age 50 years. |
| 5. CRC diagnosed in two or more first- or second-degree relatives with HNPCC-related tumors, regardless of age. |
Once a diagnosis has been made, an aggressive screening regimen is initiated including colonoscopy every 1-2 years starting at age 20-25. Women are advised to undergo endometrial cancer screening or hysterectomy if childbearing is complete. In families with a history of upper urinary tract or gastric cancers, screening for renal and ureteral cancer is initiated at age 30-35 years. A decision analysis model suggested that prophylactic total abdominal colectomy at age 25 would offer a very small survival benefit (1.8 years) and a decreased quality of life compared with colonoscopic surveillance. Therefore, surgical intervention is generally reserved for patients who develop a cancer or polyp that is unable to be removed endoscopically. However, because metachronous tumors occur in 40% of patients at 10 years an aggressive surgical approach is warranted. If a patient presents with a colon cancer, one option would be total abdominal colectomy with ileorectal anastomosis; however, even with this aggressive approach, the risk of developing a future rectal cancer is 6%-20%. Because any rectal remnant left in situ is a risk for developing a future cancer, an aggressive approach is appropriate; however, the decision regarding the specific intervention is multifactorial and depends on the patient’s preoperative continence, ability to cope with the change in bowel habits that occur after low anastomoses, and desire to participate in postoperative surveillance. If the patient prefers protectomy or presents with a rectal cancer a restorative proctocolectomy with ileal pouch anal anastomosis (IPAA) (J-pouch) can be offered if safe from an oncologic perspective. However, if the tumor precludes sphincter preservation or the patient is otherwise not a candidate for a J-pouch, a total proctocolectomy with end ileostomy is performed.
The second most common CRC syndrome is FAP. This syndrome accounts for less than 1% of all CRCs. Like HNPCC, it is autosomal dominant with nearly 100% penetrance; however, in contrast, these patients are more likely to develop left-sided CRC at an earlier age (> 90% by age 40). Twenty percent of cases of FAP are sporadic. Most are a result of a mutation of the DNA mismatch repair gene APC located on chromosome 5q21. Depending on the location and type of mutation, the phenotypic presentation can range from mild to severe. Individuals with classic FAP typically have more than 100 colonic adenomas but can have more than 1000. These develop at an early age and are present in 50% of patients by age 15. In contrast, patients with attenuated FAP (aFAP) have an average of 30 polyps that develop after age 25. Although most patients with the attenuated form develop colon cancer, the average age is 59 years.
MYH-Associated polyposis (MAP) is clinically similar to FAP and aFAP. Most patients develop a mean of 50 polyps, although cases have been documented with 5-750. However, it is distinguished by family history as it is an autosomal recessive condition caused by mutations in the base excision repair gene MYH.
There are a variety of extracolonic manifestations described in patients affected by the polyposis syndromes. Duodenal neoplasms and desmoid tumors occur in 85% and 15% of patients, respectively. These two manifestations are important because they contribute to increased morbidity and mortality in FAP patients. Esophagogastroduodenoscopy (EGD) is recommended for duodenal screening in these patients beginning at the age of 20. Desmoid tumors are commonly intra-abdominal in FAP patients. They are often found incidentally at surgery but can be aggressive and result in significant GI symptoms. Other manifestations include retinal pigment epithelium hypertrophy, osteomas, and sebaceous cysts. Gardner syndrome is applied to families with the constellation of polyposis, osteoma, dental abnormalities, and soft tissue tumors; Turcot syndrome refers to patients affected by polyposis and medulloblastoma.
Management involves genetic counseling, aggressive screening, and prophylactic colectomy. Patients at risk for FAP should undergo initial colonoscopy at the age of 12, or 20 if suspected to have aFAP. Considering the predominantly left-sided disease in FAP, it has been suggested that these patients can be followed with annual or biannual flexible sigmoidoscopy; however patients with aFAP should be followed with colonoscopy. Prophylactic colectomy is warranted in all patients, especially those with severe polyposis, dysplasia, symptoms, or large adenomas. This can be delayed if there is a mild presentation, but otherwise should be performed as soon as practical. Options are the same as those for HNPCC, and include total proctocolectomy with end ileostomy, restorative proctocolectomy with IPAA, and total abdominal colectomy with ileorectal anastomosis, again depending on preoperative bowel function and patient preference. Abdominal colectomy is reserved for patients with rectal sparing and attenuated disease, which most often occurs in the presence of a specific mutation upstream of c1250. However, the retained rectum is still at risk and these patients still require surveillance proctoscopy with resection or destruction of polyps every 6 months. More commonly, patients elect to undergo restorative proctocolectomy with IPAA. Patients who have a stapled anastomosis will retain about 1 cm of the anal transition zone (ATZ) and due to the predilection for ileal polyps, patients undergoing IPAA require lifelong surveillance. Some surgeons favor mucosectomy to reduce the risk of polyps in the ATZ but the ileal polyps are not affected and the functional outcome of a mucosectomy with handsewn anastomosis is less favorable than for those with a stapled anastomosis. Celecoxib is approved by the FDA for chemoprevention and may be a useful adjunct in these patients. Even with perfect surveillance and appropriate surgical management, FAP patients have an excess mortality related to extracolonic disease including upper GI malignancy and desmoids.
Juvenile polyposis is a rare syndrome characterized by the development of excessive non-adenomatous polyps. It is an autosomal dominant condition, most commonly associated with germline mutations in the SMAD4 or BMPR1A genes. The polyps are a distinct histologic subtype referred to as “juvenile” polyps, which are similar to hamartomas with edematous lamina propria and inflammatory changes. Although the pathway to carcinoma in these patients has not yet been fully elucidated, these patients have an elevated lifetime risk of developing CRC of 39%-68%. It is thought that carcinoma development is a result of neoplastic epithelial changes in the setting of exposure to the inflammatory stromal environment. The syndrome is diagnosed by the presence of five or more juvenile polyps in the gastrointestinal tract or the presence of any number of juvenile polyps with a family history of juvenile polyposis. There are three associated syndromes: Cronkhite–Canada syndrome (juvenile polyposis and ectodermal lesions), Bannayan–Riley–Ruvalcaba syndrome (juvenile polyposis, macrocephaly and genital hyperpigmentation), and Cowden disease (juvenile polyposis, facial trichilemmomas, thyroid cancer, goiter, and breast cancer).
These patients should undergo surveillance of the entire gastrointestinal tract for polyps beginning at the time of diagnosis or the onset of symptoms. This should continue annually in the presence of polyps. However, if the patient is polyp-free, the interval can be extended to 2-3 years. Surgery is reserved for patients with severe diarrhea, bleeding, or intussusception, those who fail endoscopic management of their polyps, exhibit dysplastic changes, and for those patients with a strong family history of CRC.
Peutz–Jeghers syndrome is a rare autosomal dominant condition (1/200,000 population) caused by a mutation in the STK11 gene on chromosome 19. The syndrome is defined by multiple hamartomas in the gastrointestinal tract (stomach to the rectum), mucocutaneous pigmentation, and elevated risk of gastrointestinal, breast, pancreatic, cervical, ovarian, and testicular cancers. The lifetime risk of CRC is reported to be 39%. Diagnosis is made in patients with two or more Peutz–Jeghers polyps or a combination of polyps, mucocutaneous pigmentation, or family history. There is insufficient evidence to suggest that the polyps themselves represent a premalignant lesion; however, they do become symptomatic in 50% of patients by the age of 20. Symptoms include anemia secondary to bleeding and abdominal pain secondary to infarction, intussusception, or obstruction. Because of the rarity of the condition, management has not been well defined. Regular endoscopic surveillance (including small bowel capsule endoscopy) is advocated for the early detection of malignancy and resection of symptomatic lesions. This is recommended to begin at age 8, with the frequency determined by the burden of hamartomas. When endoscopic removal is not possible, exploratory surgery with resection and intraoperative small bowel endoscopy is advised. These patients also must undergo appropriate screening for the extra-intestinal malignancies. Optimal regimens are under active study.
CRC screening is important for two reasons. The first is the early detection of colorectal carcinomas and the second is preventing CRC through the identification and removal of colorectal adenomas, which are cancer precursors. Trials have shown that the detection of early CRCs by surveillance reduces mortality. Similarly, patients who undergo endoscopic removal of adenomas have a 53% reduction in CRC-specific mortality at 16 years. The benefit is increased in patients with above average risk for CRC.
The most updated guidelines on CRC screening were published in 2008. This includes three separate guidelines from the American College of Gastroenterology (ACG), the United States Preventative Services Task Force (USPSTF), and a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer (which includes the American Society of Colon and Rectal Surgeons), and the American College of Radiology (Table 30–4). While they vary in certain recommendations, all agree that screening for average risk patients should begin at age 50 and should occur at regular intervals depending on the method, of which several are available. These include fecal occult blood test (FOBT), fecal immunochemical test (FIT), stool DNA, barium enema, CT colonography, flexible sigmoidoscopy, and colonoscopy. All abnormalities detected by screening tests require a colonoscopy for diagnosis and treatment. The decision of which method to use must be individualized, taking into account several factors, including sensitivity and specificity, risks, costs, patient adherence, and availability. The ACG guidelines suggest that African American men should begin screening at age 45. The USPSTF guidelines recommend screening selectively in patients between ages 75 and 85 years and not screening patients over 85 years. The USPSTF does not recommend the newer stool DNA and CT colonography tests until more performance data are available. All guidelines agree that prevention of colon cancer is the primary goal of screening and that the decision should be made on an individual basis to maximize effectiveness.
| Risk | Procedure | Onset (Age, y) | Frequency |
|---|---|---|---|
| I. Low Risk | |||
| A. Asymptomatic—no risk factors | Fecal occult blood testing and flex-sig | 50 | FOBT yearly. Flex-sig every 5 years |
| B. Colorectal cancer in none of first-degree relatives | Total colon examination (colonoscopy or double-contrast barium enema and proctosigmoidoscopy | 50 | Every 5-10 years |
II. Moderate Risk (20%-30% of people) | |||
| A. Colorectal cancer in first-degree relative, age 55 or younger, or two or more first-degree relatives of any ages | Colonoscopy | 40, or 10 years before the youngest case in the family, whichever is earlier | Every 5 years |
| B. Colorectal cancer in a first-degree relative over age 55 | Colonoscopy | 50, or 10 years before the age of the case, whichever is earlier | Every 5-10 years |
| C. Personal history of large (> 1 cm) or multiple colorectal polyps of any size | Colonoscopy | 1 year after polypectomy | If recurrent polyps—1 year |
| If normal—5 years | |||
| D. Personal history of colorectal malignancy—surveillance after resection for curative intent | Colonoscopy | 1 year after resection | If normal—3 years |
| If still normal—5 years | |||
| If abnormal—as above | |||
| III. High Risk (6%-8% of people) | |||
| A. Family history of hereditary adenomatous polyposis | Flex-sig; consider genetic counseling and genetic testing | 12-14 (puberty) | Every 1-2 years |
| B. Family history of hereditary nonpolyposis colon cancer | Colonoscopy; consider genetic counseling and genetic testing | 21-40 | Every 2 years |
| 40 | Every year | ||
| C. Inflammatory bowel disease | |||
| 1. Left-side colitis | Colonoscopy | 15th | Every 1-2 years |
| 2. Pancolitis | Colonoscopy | 8th | Every 1-2 years |
All methods have their strengths and weaknesses. Stool tests, like FIT, FOBT, and the stool DNA test are noninvasive with essentially no risks. They are more acceptable to patients as a screening modality; however, there is poor adherence in clinical trials on the part of both the physician and patient with the examination not being performed at the proper intervals or patients not undergoing colonoscopy for positive tests. The sensitivity of FOBT is poor (33%-40%), but this increased to 50%-75% with the development of the SENSA FOBT that is now the standard. Sensitivity and the false positive rate were addressed with the development of the stool DNA test and FIT, although these are substantially more expensive and, as mentioned, the stool DNA test is still undergoing clinical trials. It is important to note that these tests are not designed for prevention, but rather for early detection, as most advanced adenomas are not detected by these tests.
Radiographic options, including barium enema and CT colonography, are more sensitive for the detection of malignant and premalignant lesions. Both require full bowel preparation and have the drawback of radiation exposure; however, procedural risk is low. Barium enema has fallen out of favor as a screening test as it cannot detect small lesions, is unable to provide pathologic information and is very uncomfortable for patients. As CT colonography is still early in its development, its performance and appropriate screening intervals has yet to be defined; however it has been shown to have good sensitivity and specificity. It has a 90% sensitivity and a specificity of 86% for polyps more than 1 cm in size. For polyps more than 6 mm, sensitivity was 78% and specificity 88%. It is likely a poor test for detecting sessile adenomas. Using a cutoff of 6 mm for referral for colonoscopy, 15%-25% of patients would be referred, requiring an additional bowel preparation and the cost of another procedure. In addition, 5%-16% of patients will have an incidental extracolonic finding requiring further evaluation.
Flexible sigmoidoscopy is not often used in the United States for screening purposes. Its utility in preventing CRC is limited because it does not evaluate the proximal colon. It is therefore combined with a fecal screening test and colonoscopy is recommended if the fecal test is positive. Colonoscopic studies have shown that 30% of patients with advanced adenomas have no distal lesions and would therefore have a normal examination to the splenic flexure. This is more common in women and patients over age 60. This, in combination with the need for bowel preparation, patient discomfort, and poor reimbursement, has made this test infrequently utilized for screening purposes.
Colonoscopy at 10-year intervals is the preferred screening test in the ACG guidelines and is the final common step in every screening program for CRC. Its major drawback is poor adherence, likely related to the need for bowel preparation and sedation, which usually requires a chaperone and a day off work. It is the most expensive screening test and does have a small but real risk of complications (3-5/1000). A concern for missed lesions has prompted the development of tools for measuring quality. However, it is overall a safe and effective screening regimen.
Screening for high risk patients is individualized. Children with possible FAP should begin screening at puberty. Patients in families with HNPCC should begin at age 21. Patients with UC for more than 10 years should begin annual colonoscopy with random biopsies. Patients with a history of early (< age 60) CRC in one first degree relative are offered colonoscopy starting at age 40, or 10 years earlier than the family member’s diagnosis. The most recent ACG recommendations state that patients with a family history of CRC occurring in one family member older than age 60 may undergo screening as an average risk patient.
The term “polyp” is a morphologic term given to tissue that project into the lumen of the gastrointestinal tract and therefore is a broad term encompassing many entities, both benign and malignant. Non-neoplastic polyps account for 90% of all colonic polyps. Subtypes include juvenile, hyperplastic, and inflammatory polyps, as well as hamartomas (Table 30–5). There have been rare reports of carcinoma developing in association with hamartomas, but in general these polyps do not portend an increased risk for cancer. Neoplastic polyps, or adenomas, are the precursor lesions to the vast majority of colorectal adenocarcinomas in a well-studied “polyp to carcinoma” sequence, where colonic mucosa transforms into small tubular adenomas, increases in size, and develops high risk features prior to frank carcinoma. The genetic mechanisms behind this process have been well characterized and are discussed above. An exception to this is the more recently recognized serrated adenoma, which can be difficult to distinguish from hyperplastic polyps. These are found more commonly in the right colon and are thought to progress to carcinoma via MSI.
| Type | Histologic Diagnosis |
|---|---|
| Neoplastic | Adenoma |
| Tubular adenoma (adenomatous polyp) | |
| Tubulovillous adenoma (villoglandular adenoma) | |
| Villous adenoma (villous papilloma) | |
| Carcinoma | |
| Hamartomas | Juvenile polyp |
| Peutz–Jeghers polyp | |
| Inflammatory | Inflammatory polyp (pseudopolyp) |
| Benign lymphoid polyp | |
| Unclassified | Hyperplastic polyp |
| Miscellaneous | Lipoma, leiomyoma, carcinoid |
Adenomas, while accounting for only 10% of polyps, are common. Screening colonoscopy in asymptomatic patients detects adenomas in 25% of men and 15% of women. In autopsy series, they are detected in up to 60%. They are more common in older age with a prevalence of 30% at age 50 years and 55% at age 80. About 50% of patients with adenomas have more than one lesion, and 15% have more than two. They are predominantly found distal to the transverse colon and approximately half occur in the rectosigmoid area of the colon.
The presence of an adenoma indicates an increased risk for CRC. Further, adenomas themselves may harbor malignancy. The characteristics of the adenoma, including histology, location, shape, and size, significantly influence this risk. Adenomas are subdivided into tubular, tubulovillous, and villous subtypes and these exist on a spectrum. If followed over time, only 5% of tubular adenomas will develop into a malignancy, however, 22% of tubulovillous and 40% of villous adenomas will progress. Further, risk increases with size. Adenomas less than 1 cm harbor carcinoma only 1% of the time, however this risk increases to 10% in adenomas 1-2 cm and 45% of adenomas greater than 2 cm. Other factors include sessile (as opposed to pedunculated) polyps, location in the ascending colon, male sex, age greater than 60, and family history. Overall, the greatest risk factors for both the presence of carcinoma within a polyp and the future development of CRC are size greater than 1 cm and tubulovillous or villous histology. These have been termed “advanced adenomas.” Of note, these polyps require surgical resection more often as these features make them less amenable to endoscopic methods.
Adenomas, even in the absence of malignancy, are associated with risk of CRC. Patients found to have adenomas have a risk of developing metachronous cancers twofold to fivefold over patients without them. The risk is estimated to be 5%-10% per year. For these reasons, the consensus guidelines recommend complete excision of the adenoma plus more frequent colonoscopic surveillance. Colonoscopy is repeated at 5-10 years for 1-2 low risk adenomas, at 3 years if 3-10 low-risk or any high-risk adenomas, and less than 3 years if greater than 10 adenomas. Colonoscopy is repeated sooner if there is a question regarding the adequacy of the polypectomy.
Although techniques for endoscopic polypectomy are improving, surgery still remains an important option in the management of colorectal polyps. Surgery is indicated if complete endoscopic resection is not possible. Large polyps, sessile polyps, and those found in the distal rectum are the most challenging to manage endoscopically and result in a higher likelihood of positive margins or incomplete removal. Because up to 20% of these will harbor carcinoma, they require surgical resection. Surgery is also indicated in some completely resected polyps found to contain invasive adenocarcinoma.
Carcinoma is found in 5% of benign appearing endoscopically resected polyps. In 1985, Haggitt reported that depth of invasion was the most important indicator of metastasis and developed a classification system based on depth of invasion (Table 30–6). Level 1, through 4 (limited to proximal two-thirds of submucosa) with favorable histology have a < 1% risk of nodal metastasis and are adequately treated by endoscopic resection with a margin > 2 mm. However, carcinomas exhibiting evidence of vascular or lymphatic invasion, indeterminant margins, a margin < 2 mm, or level 4 with invasion into distal third of submucosa have a 12%-25% chance of lymph node involvement and therefore require formal oncologic resection.
| Level | Depth |
|---|---|
| 0 | Carcinoma in situ or intramucosal carcinoma |
| 1 | Carcinoma invading through muscularis mucosa into the submucosa but limited to the head of the polyp |
| 2 | Carcinoma invading the neck of the polyp |
| 3 | Carcinoma invading any part of the stalk |
| 4 | Carcinoma invading into the submucosa of the bowel wall below the stalk of the polyp but above the muscularis propria (T1) |
| Sessile | By definition, equivalent to level 4 |
The majority of patients with CRC are asymptomatic. Based on a median doubling time of 130 days, it takes at least 5 years, and often 10-15 years, before a cancer causes symptoms. When symptoms do appear, they are variable, non-specific, and often indicate an advanced lesion or complications. CRCs may cause subclinical bleeding, resulting in an asymptomatic iron deficiency anemia. For this reason, any iron deficiency anemia in a male or nonmenstruating female should prompt a work up to rule out bleeding from the GI tract. However, one-third of patients with CRC have normal hemoglobin at the time of diagnosis. A minority of patients will present emergently with acute obstruction, perforation, or significant bleeding with symptomatic anemia.
Compared with the left colon, tumors of the right colon may reach a more advanced stage before they cause symptoms. Obstruction is rare because the right colon is larger in diameter and has liquid stool. If these tumors bleed, melena, or more commonly occult blood, will be present in the stool. There may be associated vague abdominal pain. Ten percent of patients present with a palpable abdominal mass.
In contrast, the left colon and rectum has a smaller diameter and semisolid feces. Tumors here can cause luminal narrowing and obstruction resulting in narrowing of the stool, constipation, and increased frequency of bowel movements. Rectal cancer can produce tenesmus. When bleeding occurs, it tends to be dark to bright red and may streak or be mixed with the stool. Any patient with ongoing hematochezia, even in the presence of another clinical explanation, such as hemorrhoids, must undergo evaluation for CRC.
Physical examination may help localize and determine the extent of disease. The abdomen should be palpated for masses and the liver examined to rule out enlargement. Auscultation of the lungs is poorly sensitive for metastatic disease. A digital rectal examination may detect a distal rectal cancer. Its location, size, and mobility should be noted. Retrorectal nodes or drop metastases in the Pouch of Douglas (Blumer shelf) may be palpated. Rigid proctoscopic examination gives an accurate location of the tumor, which is important for treatment planning. Lymph node examination may reveal metastasis in the supraclavicular nodes.
The preoperative work up for CRC will determine a clinical stage, allow initial prognostics, and inform the treatment plan. It consists of laboratory tests, radiographic imaging, and endoscopy with biopsies.
Useful laboratory tests include a complete blood count, serum chemistries, urine analysis, and liver function tests as clinically indicated. These will detect anemia and other abnormalities that may need to be addressed prior to initiating treatment. The serum marker for CRC is carcinoembryonic antigen (CEA). It is a glycoprotein found throughout the gastrointestinal tract as well as other tissues. It is not sensitive or specific for patients with CRC, specifically for patients without metastatic disease. It is therefore not recommended as a screening test; however, it has been shown to be a useful adjunct to postoperative monitoring for recurrence, specifically in patients with an elevated preoperative CEA that returns to normal after surgery.
Colonoscopy is the gold standard for the diagnosis of CRC. Complete colonoscopy is indicated in all patients who have suspected or known CRC. It allows tissue diagnosis, localization of the lesion using tattoo, and evaluation for synchronous neoplasms. Three percent of patients with a known colon or rectal cancer have a synchronous lesion. In patients with obstructing lesions, the remaining colon should be evaluated using contrast enema or CT scan. These patients should undergo postoperative surveillance as soon as feasible after their surgery.
CRC generally grows circumferentially. It takes approximately 1 year for a tumor to encircle three-fourth of the circumference of the bowel wall. This is especially true in the left colon which is smaller in diameter. Submucosal extension through the lymphatic network rarely extends beyond 2 cm from the tumor. As the tumor extends radially, it may penetrate the wall and extend into neighboring structures including the liver, greater curvature of the stomach, duodenum, small bowel, pancreas, spleen, bladder, kidney, ureter, and abdominal wall. Rectal tumors in particular may invade the vaginal wall, bladder, prostate, sacrum, or levators due to the confined space of the pelvis. The inflammatory response incited by extension of the tumor is indistinguishable from frank invasion during gross examination at the time of surgery.
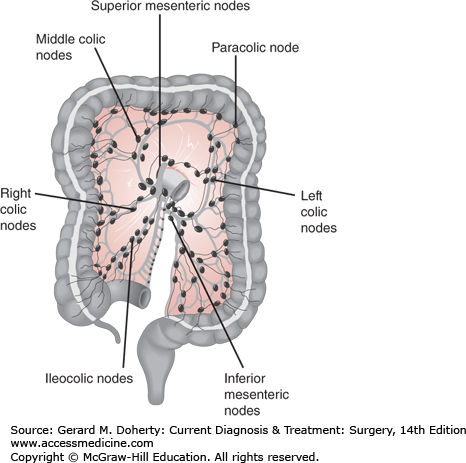
Metastatic disease most often occurs through the lymphatics, although it is also known to occur via seeding, intraluminal spread, or hematogenously. Rectal cancer metastasizes proximally to the mesorectal, iliac, and inferior mesenteric lymph nodes, as well as radially along the pelvic side walls where obturator nodes can become involved. Very distal rectal cancers can also spread to inguinal lymph nodes. Colon cancer spreads along the superior and inferior mesenteric lymph node basins (Figure 30–8). Approximately half of patients undergoing surgery for CRC will have lymph node involvement. Hepatic and pulmonary metastases occur via hematogenous invasion. In colon cancer this may occur through the portal system to the liver, and less commonly the lumbar and vertebral veins to the lungs and other organs. Metastases to ovaries are mostly hematogenous; they are found in 1%-10.3% of women with CRC. Rectal cancer spreads through the systemic circulation via the hypogastric veins.
The “no touch” technique, while never definitively shown to reduce metastatic disease or improve survival, is one of the basic tenets of surgical management of colon cancer. The concept is to minimize manipulation of the tumor prior to ligation of the blood supply in order to avoid tumor embolus and subsequent metastasis. Transperitoneal metastasis or peritoneal “seeding” may occur when a tumor has extended through the serosa leading to peritoneal implants or generalized carcinomatosis. When found in the Pouch of Douglas, these deposits are palpated on digital rectal examination and referred to as Blumer’s shelf.
Accurately staging patients with CRC is important as it allows the development of an adequate treatment plan and determination of prognosis. The clinical stage is determined using preoperative imaging. Pathologic staging is based on imaging and information obtained after resection.
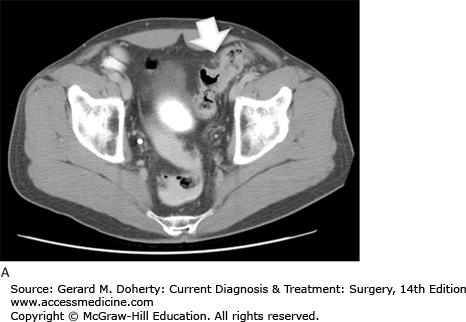
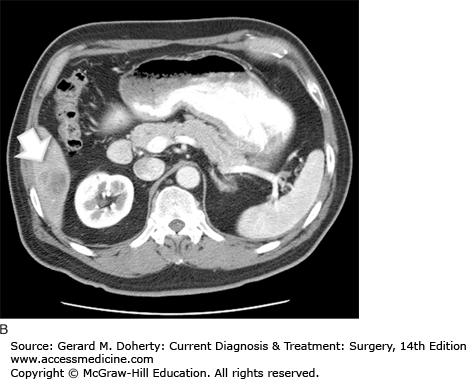
The initial determination required for developing a treatment plan for a patient with colon cancer is whether the tumor is resectable. This can be determined by searching for metastatic disease and determining local invasion. CT of the abdomen and pelvis is used routinely for this purpose. It may reveal lymphadenopathy, hepatic metastases, as well as evidence of obstruction, perforation, or direct extension (Figure 30–9). It has a sensitivity of about 78% for metastatic disease. Metastatic disease to the lungs is most often assessed using a routine preoperative chest x-ray. CT can be used to further evaluate abnormalities; however, it is not used as an initial test because these patients generally do not develop pulmonary metastases without first developing liver disease. This is because the lymphatic drainage of the colon follows the portal circulation. Positron emission scanning (PET) is not routinely recommended, however it is indicated in patients with suspicious abnormalities on CT that require evaluation for proper treatment planning.
Surgical planning for rectal cancer requires detailed evaluation of the depth of invasion of the rectal wall as well as lymph node involvement and invasion of nearby pelvic structures including the levators, sphincter complex, and genitourinary tract. This information is used to make decisions about neoadjuvant therapy, sphincter sparing procedures, and local excision (LE). Endorectal ultrasound (ERUS) and pelvic magnetic resonance imaging (MRI) are commonly used. ERUS is particularly useful for determining the depth of invasion of the rectal wall and lymph node involvement. MRI is used to detect involvement of the mesorectal fascia and nearby pelvic structures to determine the likelihood of a circumferential resection margin, which is an important predictor of local recurrence. CT and PET CT are often used to assess pulmonary and liver metastasis, although the utility of this imaging modality requires further study.
The standard staging system in the United States is the tumor, node, metastasis (TNM) staging system from the American Joint Committee on Cancer (AJCC). This has replaced the Dukes classification. These are shown in Table 30–7. The clinicopathologic stage is the most important determinant of survival. Stage for stage, patients with colon cancer have a better prognosis than patients with rectal cancer. Similarly, patients with proximal rectal cancers have a better prognosis than those with distal cancers. Poor prognostic factors include complications such as obstruction or perforation, as well as histologic features such as poor differentiation and lymphovascular or perineural invasion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree