Laparoscopic Splenectomy
Steven D. Schwaitzberg
History
While open splenectomy has been performed since the 1500s, reports of laparoscopic splenectomy began appearing in the literature in 1991 and 1992. Over time it has become the de facto standard for the performance of elective splenectomy for both benign and malignant indications although incisional (open) splenectomy remains perfectly acceptable for all indications as well. This technique was initially proposed for normally sized spleens but recent reports demonstrating consistent success utilizing hand-assisted techniques and the utilization of larger extraction bags make removal of very large spleens feasible using minimally invasive techniques as well. The most recent technique adaptations involve the use of robotics to perform the resection. The benefits of minimally invasive resection of the spleen are similar to those of other solid organ resections (adrenal, pancreas, liver, kidney), allowing for smaller less painful incisions, improved cosmesis, shorter hospitalization, earlier return to full activity, and a reduction of a variety of pulmonary and wound complications.
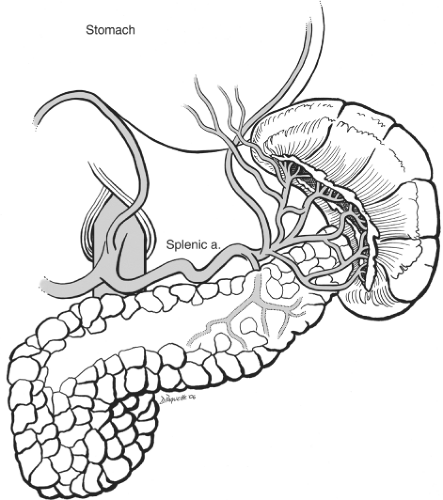
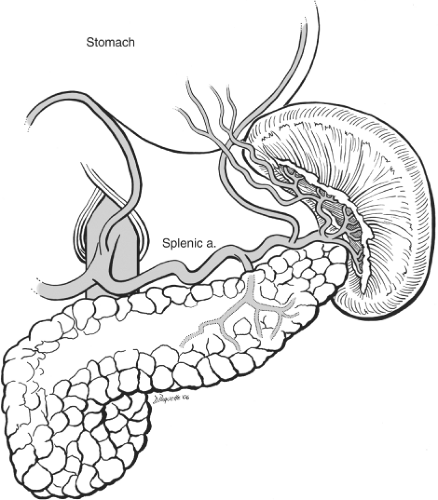
The normal spleen is a 100- to 200-g bluish purple, wedge-shaped structure residing in the uppermost posterior region of the left upper quadrant. It is probably an evolutionary survival benefit that this friable and somewhat fragile, vascularly rich solid organ is protected by the left 9th, 10th, and 11th ribs. The corollary to this is that injury to these rigid bony structures is associated with a significant incidence of concomitant splenic injury. The superior, anterior, and lateral borders of the spleen are bounded by the diaphragm and ribcage. The splenic flexure of the colon rests at the tip of the lower pole. The upper pole of the kidney and the adrenal general rest just posterior to the lower pole as well. Medially the tail of the pancreas is found at the level of the hilum with 30% of spleens abutting this structure and the rest within 2 cm. Also, medial but slightly more anterior are the short gastric vessels leading to the proximal greater curvature of the stomach. Since splenectomy is essentially the act of dividing the relevant vascular and ligamentous attachments the surgeon must be familiar with these structures. The spleen is supplied by two sets of vascular structures: the short gastric vessels and the splenic artery/vein. The three to five short gastric vessels drain the fundus and left part of the greater curvature of the stomach, and pass between the two layers of the gastrosplenic ligament to end generally in the superior splenic vein branches. There is often a direct connection between one of the short gastric vessels and a superior polar branch of the splenic artery. In addition, there can be a branch from the left gastroepiploic artery to the inferior polar branches of the splenic artery. The splenic artery anatomy is highly variable and like snowflakes no two spleens have quite the same anatomic vascular arrangement. There are, however, two major configurations described: distributed and magistral as originally described by Michels in 1942. The distributed supply (Fig. 1) is commonly characterized by a shorter main splenic trunk and relatively early branching resulting in several relatively long branches with numerous transverse connections between them. These branches fan out over and cover a generous portion of the hilar surface. The magistral supply is notable for a longer main splenic trunk with relatively late and more limited terminal branches covering less of the hilum region and was well described by Poulin in 1993 (Fig. 2). In general, the splenic vein branches are posterior or behind the arteries although this arrangement becomes more variable closer to or within the spleen. A recognition of the nature of the splenic artery distribution will lead to a safer and more efficient dissection of the hilum. By looking at the notches and tubercles on the surface of the spleen one can generally correlate the number of branches entering the spleen. In addition, there are branches from the splenic artery to the tail of the pancreas. Therefore, it is important to understand that when indicated, it is preferable to ligate or embolize the splenic artery distal to pancreatic
branches to avoid possible pancreatic injury from ischemic injury post operatively.
branches to avoid possible pancreatic injury from ischemic injury post operatively.
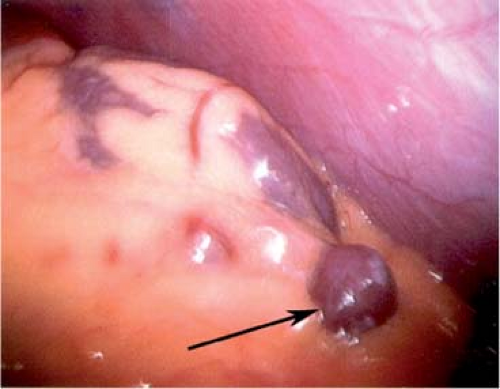
The spleen is suspended by a series of ligaments: gastrosplenic, lienorenal, splenocolic, phrenosplenic, and splenorenal. The first two contain the blood supply. The latter three are reflections of the peritoneum that cradle the spleen from the superior pole laterally to the inferior pole. They are generally avascular unless portal hypertension is present. The spleen develops around the 5th week of gestation (6- to 8-mm fetus). Mesodermal elements close to the pancreas fuse to form the spleen. Imperfect fusion of these elements results in notching or lobulation of the spleen. Complete failure of one of these elements to fuse will result in the formation of an accessory spleen. The majority of these accessory spleens are found in the splenic hilar region but may be located throughout the abdomen and even occasionally in the pelvis (Fig. 3).
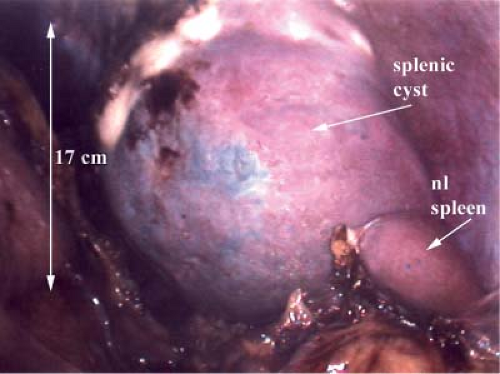
While case reports of splenic rupture managed laparoscopically exist, this technique is generally reserved for elective indications. The most common indication is idiopathic thrombocytopenia purpura (ITP) that is refractory to medical management. This is an ideal indication since patients with ITP do not present with splenic enlargement. Other hematologic indications include selected patients with thalassemia, hereditary spherocytosis, hairy cell leukemia, myelofibrosis, or hereditary elliptocytosis. Felty’s syndrome is an uncommon manifestation of seropositive rheumatoid arthritis associated with granulocytopenia and splenomegaly. Splenectomy is indicated when refractory granulocytopenia is present. Storage diseases such as Gaucher’s disease remain an uncommon indication for surgery when enzyme replacement therapy fails to improve life-threatening thrombocytopenia. Splenic cysts and abscesses can be removed via a laparoscopic approach (Fig. 4). Minimally invasive splenectomy can be considered for nearly any indication, but the surgeon is well advised to consider operative acumen in terms of the splenic size and etiology before determining the approach to the spleen. Hypersplenism secondary to cirrhosis of the liver is an occasional indication for splenectomy. In this instance laparoscopic resection can be performed, but only by experienced operators. In terms of size as a driver of operative access, the interpolar length of the normal spleen typically measures 7 to 11 cm. An experienced operator can resect these spleens as well as moderately enlarged ones up to 20 cm laparoscopically usually without a hand-assisted technique. Massively enlarged spleens can still be resected minimally invasively but it is generally more feasible to do this utilizing a hand-assisted technique. Splenic size can be evaluated by ultrasound or computed tomography (CT); the latter may be more useful in detecting accessory spleens. If doubt remains concerning the presence of accessory spleens, then a liver/spleen nuclear medicine scan may be helpful in this evaluation.
This first step in the preoperative phase is a careful counseling session with the patient. Creating the appropriate patient expectation preoperatively may avoid problems or unexpected disappointment from the patient postoperatively. Surgeons should give the patients a reasonable incidence of “conversion rates” to incisional surgery. While the surgical community does not view a “conversion” as a complication, the patient may have a different view unless properly educated. It is convenient to divide complications into four categories: those due to surgery, those due to laparoscopy, those due to operative splenectomy, and those due the nature of the treatment (Table 1). Surgeons will vary when advising prospective patients concerning the precise incidence of these events, but operators should be able to perform this procedure with less than 1% mortality and less than 4% incidence or pancreatic injury. Clearly, splenectomy is not uniformly successful in all cases of ITP. Approximately 65% to 75% of patients will resolve their thrombocytopenia. Patients who are refractory to steroids and immune globulin therapy may have even less success with splenectomy. Patients need to be educated as to the risks that the asplenic patient engenders postoperatively in terms of infection. Overwhelming postsplenectomy sepsis is uncommon in adults; however, patients who consent to the procedure should be vaccinated against certain types of Gram-positive bacteria. Polyvalent pneumococcal vaccine should be administered at least 2 weeks prior to splenectomy. In addition, Haemophilus influenzae type b (Hib) conjugate vaccine and meningococcal serogroup C conjugate vaccine should administered as well. Even with vaccination, patients should be advised that should procedures associated with potential bacteremia be contemplated in the future, antibiotic prophylaxis is indicated. Patients should also be reminded about the need to communicate to health care providers taking a history that a splenectomy has been performed in the past. Patients often ask whether or not revaccination is needed. The answer to this is not clear. Some patients demonstrate low antibody levels to capsular polysaccharide antigens after 5 to 10 years has lapsed postsplenectomy. When this occurs revaccination can be performed safely. Finally, to the extent that the risks and benefits have been outlined and vaccinations administered, there is a need to document this and make sure the patient has this information as well, including which vaccines have been given and the specific prophylactic antibiotic recommendations.
Table 1 Potential Complications of Laparoscopic Splenectomy that Should Be Reviewed in the Preoperative Discussion | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Splenic artery embolization has become less popular in the last decade. Despite this, some surgeons may find this useful when attempting to remove a very large spleen or as an adjunct to partial splenectomy. Care to preserve the blood supply to the distal pancreas should be exercised by the angiographer (Fig. 5). Embolism can be performed at two levels along the splenic artery and ideally the surgeon will conduct the dissection in between these sites for maximum hemostasis. When this adjunct is used it should be performed just prior to splenectomy, ideally on the same day. Infarction of the splenic mass can be extremely painful to the patient and this should be anticipated in terms of counseling and the availability of pain medicine.
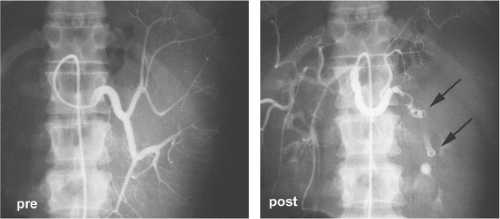 Fig. 5. Angiographic pre- and postembolization views of splenic artery with double-coil technique (arrows). |
Just after the patient enters the operating room, a single dose of antibiotic prophylaxis against Gram-positive organisms should be administered. Since many of these patients are on steroids, the plan for administering “stress doses” of steroids should be discussed with the anesthesia team. While the immediate availability of blood products and platelets must be ensured prior to beginning the procedure, it is generally not necessary to actually administer platelets unless a bleeding diathesis is encountered. The old practice of “hanging platelets with the incision” does not appear to improve outcome or reduce blood loss in laparoscopic splenectomy. Sequential compression devices should be placed on the patient’s lower extremities in order to minimize the risk of deep venous thrombosis. Urinary and (oro)nasogastric catheters are placed for decompression. Splenectomy is an advanced laparoscopic procedure requiring the dissection and control of several vascular structures. As such, the surgeon must have the appropriate tools immediately at his/her disposal. I have found that, in addition to the usual array for graspers, a 10-mm right angle dissector and a DeBakey-type noncrushing grasper are particularly useful while performing the dissection. Many surgeons control the main splenic vessels with linear endoscopic staplers. The surgeon should confirm the presence and the number of “vascular loads” available for that device prior to initiating the procedure since halfway through the operation is the wrong time to find out that supplies might be short.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree