Laparoscopic Radical Prostatectomy
Jeffrey A. Cadeddu
J. Kyle Anderson
Introduction
Prostate cancer is the most common nonskin cancer and the second largest killer in men among all malignancies. In 2009 there were an estimated 192,280 new cases diagnosed and 27,360 deaths from prostate cancer. Given the scope of prostate cancer, it is little surprise that multiple treatment options exist for biopsy-proven prostate cancer. Currently these include watchful waiting, active surveillance, hormone therapy (gonadotropin releasing hormone agonists or orchiectomy), ablative technologies (cryotherapy or high-intensity focused ultrasound), brachytherapy, external beam radiation therapy, open radical perineal prostatectomy, open radical retropubic prostatectomy, robot-assisted radical prostatectomy (RARP), and laparoscopic radical prostatectomy (LRP). Of these options, the various techniques for prostatectomy provide the most definitive treatment. Not only is the entire gland removed but surrounding lymph nodes are obtained in select cases. This combination provides both pathologic staging and prognostic information, and for the open prostatectomy, it has a long track record as an unsurpassed treatment for localized prostate cancer.
Open radical prostatectomy (ORP) was first reported as a treatment for prostate cancer in 1905 by Hugh Hampton Young. Initial technique was perineal, with incision anterior to the anus and dissection retrograde toward the bladder neck. A major innovation came with the anatomic retropubic approach pioneered by Walsh in the 1970s. This technique provided much improved vascular control of the dorsal venous complex, superior dissection of the prostatic apex, and preservation of the neurovascular bundles responsible for erections. Postoperative incontinence and impotence had always been the major deterrents to ORP, but with preservation of the external urethral sphincter and neurovascular bundles these complications were greatly reduced. More recent improvements in prostatectomy techniques have included LRP. Schuessler et al. performed the first LRP in 1991. The technique was further described by two French groups Guillonneau and Vallancien at Montsouris Hospital and Abbou et al. at Henri Mondor Hospital. With their work, LRP allows removal of the prostate with less blood loss, shorter postoperative convalescence, and comparable oncologic effectiveness when compared with open surgical approaches. Additionally, it provides a procedure comparable with RARP without the expense of a surgical robot. In this chapter, we will cover the important technical points of LRP and briefly discuss surgical outcomes relative to open radical retropubic prostatectomy.
Prostatectomy is suitable for patients with at least 10 years’ life expectancy and medical conditions suitable for a major operative procedure. Diagnosis of prostate cancer should be documented with appropriate biopsies, and preoperative clinical staging workup (digital rectal examination and prostate-specific antigen [PSA] level) should demonstrate organ-confined disease. Additionally, bone scan and abdominal computed tomography scan for patients with Gleason score of 8 or above or PSA >20 should be obtained. Although prostate size on either extreme (below 20 g or above 80 g) can increase the difficulty of the procedure, there is no absolute limit on gland size that can be removed. Neoadjuvant hormone ablation does not improve survival and can increase periprostatic adherence of tissues,
and thus it should be avoided if possible. LRP following previous radiation treatment to the pelvis is not well described and is not recommended. Finally, the question of nerve-sparing prostatectomy to preserve potency must be addressed with the patient preoperatively. Relative contraindications to nerve sparing are impotence, large nodule palpable at the prostatic apex, locally advanced disease, and PSA >20 ng/mL. Intraoperatively, adherence of the neurovascular bundles to the prostate or unusual thickness of the bundles would be additional contraindications to the nerve-sparing technique.
and thus it should be avoided if possible. LRP following previous radiation treatment to the pelvis is not well described and is not recommended. Finally, the question of nerve-sparing prostatectomy to preserve potency must be addressed with the patient preoperatively. Relative contraindications to nerve sparing are impotence, large nodule palpable at the prostatic apex, locally advanced disease, and PSA >20 ng/mL. Intraoperatively, adherence of the neurovascular bundles to the prostate or unusual thickness of the bundles would be additional contraindications to the nerve-sparing technique.
Additional contraindications specific to LRP are few. Patients with previous extensive intra-abdominal surgery or history of peritonitis are better served by alternative modalities or an extraperitoneal approach. Additionally, a history of cerebral vascular abnormalities (aneurysms, arteriovenous malformations, or cerebral vascular accidents) may increase the risk of complication given the extreme Trendelenburg position needed for this procedure. Obesity is a relative contraindication as a large pannus can markedly change instrument angle of entry into the abdomen and make intra-abdominal manipulation much more difficult. Finally, pelvic lipomatosis, previous open bladder, or previous prostate surgery are relative contraindications to this procedure.
 Surgical Technique, Postoperative Management, and Complications
Surgical Technique, Postoperative Management, and ComplicationsThere are two described approaches to LRP: intraperitoneal and extraperitoneal. Choice of approach should be surgeon preference, as differences in outcomes between the two techniques are not well proven. Of the two, the intraperitoneal approach is more commonly described and is probably easier to master given the larger working space and easy access to the seminal vesicles. Thus, it is the intraperitoneal procedure that we will describe first. The intraperitoneal LRP with nerve sparing can be broken down into a number of steps including placement of trocars, dissection of the vas and seminal vesicles, mobilization of the bladder, opening of the endopelvic fascia, ligation of the dorsal venous complex, bladder neck dissection, preservation of the nerves, division of the prostatic pedicle, dissection of the prostatic apex, removal of the prostate, and urethrovesical anastomosis. Minor deviations from this approach have been reported and will be mentioned where appropriate. Differences between the intraperitoneal and extraperitoneal approach will be highlighted as well, although once the respective space has been accessed, the two procedures are quite similar.
Surgical preparation begins the day before surgery with a magnesium citrate bowel preparation. This decompresses the colon (as colonic or rectal distension can greatly hinder exposure) and allows primary repair in the unlikely event that a rectal injury occurs. On the day of surgery, an intravenous first-generation cephalosporin antibiotic is administered preoperatively, two or more intravenous access points are obtained, and sequential compression devices are applied to the lower extremities.
Upon entering the operating room, general anesthesia is induced and the patient is positioned with the umbilicus over the kidney rest. We prefer straps or tape placed in an “X” over the patient’s shoulders and chest in order to prevent slippage when the patient is placed in deep Trendelenburg position later in the procedure. An oral or nasal gastric tube is placed for decompression of the stomach, and, as always, during laparoscopy, nitrous oxide inhalation gas should be avoided. A rectal bougie may be placed at this time for later manipulation of the rectum, but we have not found this necessary. We do, however, spread the legs to allow access to the perineum later in the procedure. Finally, an important goal for the anesthesiologist is to minimize intravenous fluids. With the steep Trendelenburg position required for this procedure, facial and laryngeal edema and subsequent difficulty removing the endotracheal tube is a significant risk.
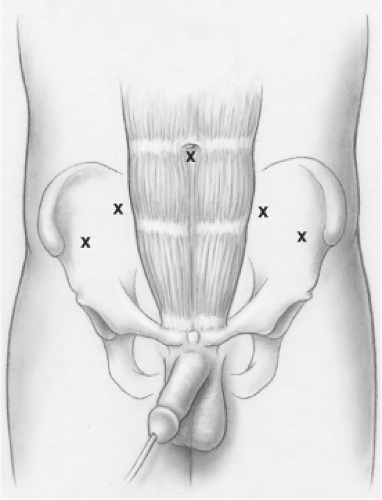
With sterile preparation and draping complete, an 18 French Foley catheter is inserted into the bladder. A transverse incision is made immediately cranial to the umbilicus and the intraperitoneal space is inflated to a pressure of 15 mm Hg. A 12-mm trocar is placed at this site and a 0-degree telescope is inserted. This is the only lens necessary for the surgery. Four more trocars are placed as shown in Figure 1. The medial two ports are 12 mm in size and are located 4 to 5 cm lateral and caudal to the umbilical trocar. The two lateral ports are 5 mm in size and are placed 4 to 5 cm laterally and slightly caudally from the 12-mm trocars. Once the trocars are in position, the bed is placed in maximal Trendelenburg position (approximately 30 degree head down).
These trocar locations allow the operating surgeon to work through the two ports on the left side, and the assistant to function autonomously via the two right-side trocars. Visualization is provided almost exclusively through the umbilical port with a 0-degree telescope. A second assistant can operate the camera from a position at the head of the bed, or alternatively a robotic arm or other positioning device can be mounted on the right bed rail and used for camera control. Monitors are positioned at the foot of the bed with one monitor per side. Cautery and harmonic scalpel (if used) are placed at the foot of the bed as well. Instrumentation utilized during LRP includes monopolar cautery scissors, bipolar graspers, tissue graspers, suction irrigation device, needle drivers, and ultrasonic shears.
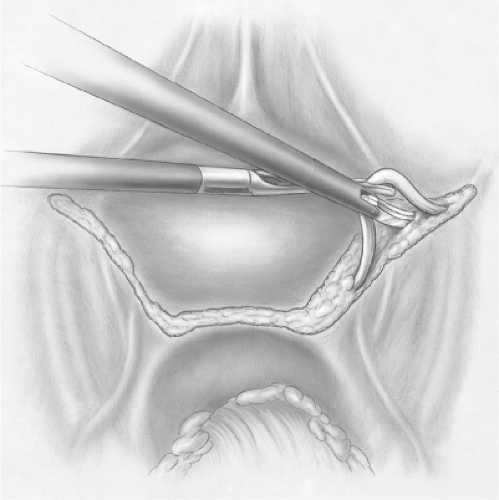
The surgeon begins the procedure with grasper and monopolar scissors in the left-sided ports. The assistant utilizes a blunt tip grasper in the medial port and suction irrigator in the lateral port. Frequently on entering the pelvis, adhesions of the sigmoid colon are encountered. These are easily taken down and the rectovesical pouch is well visualized. A thorough anatomical understanding of the posterior aspect of the prostate and seminal vesicles is important given that structures to be avoided (namely rectum and ureters) are in close proximity. Figure 2 shows the initial peritoneal incision as well as the relationship of the seminal vesicles to the prostate and ureters. With the assistant applying upward retraction with the suction irrigator and sigmoid colon retraction with the grasper, the peritoneum overlying the seminal vesicles and vas deferens is opened. The vas are the most easily identified structure and can be traced medially from the internal ring. Awareness of the ureters, which lie anterior and lateral from the vas, is important as the
potential for ureteral injury exists. The peritoneum over the vas is opened, the vas are dissected free for 3 to 4 cm, and each is divided. Dissection of the seminal vesicles is undertaken with the assistant providing anterior and posterior retraction at the respective peritoneal edges. Progress is made toward the tip of the seminal vesicle where the vascular supply is encountered and controlled, generally with clips. During a nerve-sparing procedure, care must be taken when approaching the tips of the seminal vesicles as the neurovascular bundle is in close proximity and the use of cautery can have a deleterious effect on the nerves. Once both seminal vesicles and vas are free, these structures are retracted anteriorly and Denonvilliers’ fascia is opened. The space between the prostate and anterior rectal wall is developed, as this will greatly simplify later dissection of the prostatic pedicles.
potential for ureteral injury exists. The peritoneum over the vas is opened, the vas are dissected free for 3 to 4 cm, and each is divided. Dissection of the seminal vesicles is undertaken with the assistant providing anterior and posterior retraction at the respective peritoneal edges. Progress is made toward the tip of the seminal vesicle where the vascular supply is encountered and controlled, generally with clips. During a nerve-sparing procedure, care must be taken when approaching the tips of the seminal vesicles as the neurovascular bundle is in close proximity and the use of cautery can have a deleterious effect on the nerves. Once both seminal vesicles and vas are free, these structures are retracted anteriorly and Denonvilliers’ fascia is opened. The space between the prostate and anterior rectal wall is developed, as this will greatly simplify later dissection of the prostatic pedicles.
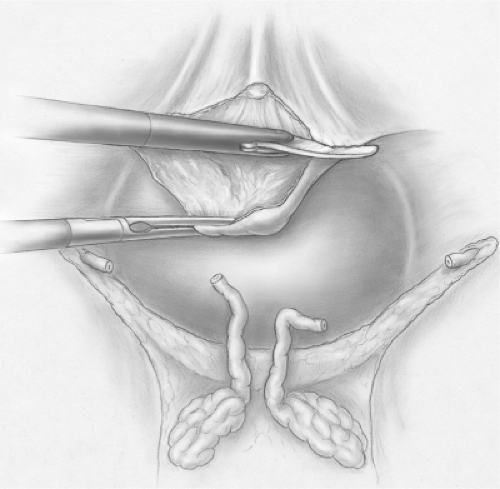
The next component of the procedure is the mobilization of the bladder and opening of the endopelvic fascia (Fig. 3). To begin, the bladder is filled with 200 mL of saline to aid in identification and dissection of the bladder from the anterior abdominal wall. Initial incision can be medial or lateral to the medial umbilical ligament, but, regardless, awareness of the external iliac vein location is important as it is frequently in close proximity. The most important landmark is the pubic bone. Once this is identified, the space of Retzius is entered and the bladder is mobilized posteriorly.
With the bladder dropped posteriorly, adipose tissue overlying the endopelvic fascia and prostate is removed. The endopelvic fascia is easily opened, and with sharp and blunt dissection, levator muscle fibers are separated from the prostate. Dissection is continued to the prostatic apex and when the superficial dorsal vein and puboprostatic ligaments are encountered, these structures are cauterized and divided. Control of the dorsal venous complex at the prostatic apex is next accomplished with intracorporeal stitch placement and knot tying using a figure of eight 0-Vicryl stitch on a CT-1 needle. This stitch should be deep enough to encompass the complex but should not enter into the urethra (Fig. 4). Division of the dorsal venous complex is delayed until later in the procedure, as division at this time results in increased blood loss.
The next important maneuver is the separation of the base of the prostate from the bladder neck (Fig. 5). Correct identification of the plane of dissection is critical to achieve a satisfactory bladder neck; however, this plane is not always obvious. Thus, a number of techniques have been developed to identify the junction between the prostate and the bladder. First, the adipose tissue is generally easily removed from the prostate using blunt dissection. This is not the case with the bladder, and thus this change in adipose tissue adherence marks the vesicoprostatic junction. Next, the Foley catheter may be exchanged for a Van Buren sound. By angling the tip of the sound anteriorly while passing the sound through the bladder neck, the junction between bladder and prostate becomes quite obvious. Alternatively, movement of the Foley catheter balloon against the bladder neck highlights the junction. Additional information about the presence of a median lobe is also gleaned from this final maneuver, as a large median lobe will often cause the balloon to deviate to one side. Once the junction is identified, a 2-0 Vicryl stitch on an SH needle is placed in the midline just on the prostatic side of this junction. With the assistant providing anterior traction on this stitch through the 5-mm lateral port and frequent suction through the 12-mm port, the surgeon is able to divide the bladder
away from the prostate. Previously we used ultrasonic shears for this portion of the procedure but with increased experience monopolar cautery scissors provide a sufficient and more cost-effective tool. The direction of incision is almost directly posterior, despite the initial appearance of the prostate, which would suggest a more caudal direction. If a median lobe is present, the mucosa along the posterior edge of the lobe is divided and the median lobe mobilized away from the bladder and along with the remainder of the prostate. If the median lobe is absent, the division of the prostate from the bladder is continued posteriorly (Fig. 6). Once the posterior aspect of the bladder has been separated from the prostate, the seminal vesicles and vas are visible and can be pulled through the opening between the bladder and the prostate.
away from the prostate. Previously we used ultrasonic shears for this portion of the procedure but with increased experience monopolar cautery scissors provide a sufficient and more cost-effective tool. The direction of incision is almost directly posterior, despite the initial appearance of the prostate, which would suggest a more caudal direction. If a median lobe is present, the mucosa along the posterior edge of the lobe is divided and the median lobe mobilized away from the bladder and along with the remainder of the prostate. If the median lobe is absent, the division of the prostate from the bladder is continued posteriorly (Fig. 6). Once the posterior aspect of the bladder has been separated from the prostate, the seminal vesicles and vas are visible and can be pulled through the opening between the bladder and the prostate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree