Laparoscopic Cholecystectomy, Intraoperative Cholangiography, and Common Bile Duct Exploration
John G. Hunter
Thai H. Pham
History
In 1882, Carl Langenbuch performed the first open cholecystectomy for gallstone disease. In 1985, Eric Muhe in Boblingen, Germany, and in 1987 Philippe Mouret in Lyon, France, performed the first laparoscopic cholecystectomies (LC) in the world. Working independently, two groups of American surgeons performed LC in 1988. In 1990, 10% of cholecystectomies were being performed laparoscopically in the United States. This figure has risen dramatically over the past two decades and stands at 88% in 2006. Never before had a surgical revolution occurred so quickly. LC was a significant advance by improving recovery, which allowed patients to have better quality of life in the immediate postoperative period. Although prospective randomized trials came late; they slowed the clear advantages of LC over open cholecystectomies: less pain with faster recovery. This cataclysmic change took its toll. Training of surgeons to perform the procedure was frequently inadequate and early complications gave birth to the phrase learning curve. It is the purpose of this chapter to review the lessons of the last 20 years and to detail current techniques for LC, intraoperative cholangiography, laparoscopic ultrasonography, and common bile duct (CBD) exploration.
The major indication for LC is symptomatic cholelithiasis. Classic symptoms present in the majority of patients include stabbing (colicky) pain in the right upper quadrant radiating to the back and to the shoulder. Although it is often taught that this pain occurs after the patient eats a fatty meal, the pain pattern is not always so predictable. Frequently, the pain occurs in the middle of the night, usually between the hours of 12 a.m. and 3 a.m. After waking the patient, the pain generally lasts from a few minutes to a few hours. Usually, the pain passes within an hour, and the patient returns to sleep. Frequently, the pain is associated with nausea and vomiting but is rarely accompanied by jaundice, fevers, or chills, unless acute cholecystitis or cholangitis is present. When the pain is most severe and unrelenting, an emergency room visit is prompted. Myocardial infarction is often suspected, but it is “ruled out” with a normal electrocardiogram and cardiac isoenzymes. If a sonogram demonstrates cholelithiasis in this setting, elective LC is indicated. Occasionally, the presentation is less characteristic of biliary colic. The patient may only complain of a nagging, dull pain in the right side, epigastrium, or back without the severity of colic. In this setting, the association of gallstones and right upper quadrant pain that cannot be attributed to another cause merits consideration of LC.
Complicated gallstone disease is usually managed laparoscopically in the same manner as it was managed during the era of open cholecystectomy. Acute cholecystitis (fever, leukocytosis, right upper quadrant tenderness, and gallstones) demands more immediate attention than biliary colic. LC in patients with acute cholecystitis may be significantly more difficult and associated with conversion rates of 5% to 10% (depending on surgeon experience), but it is nonetheless worth pursuing in patients without severe concomitant risk factors.
During initial hospitalization, biliary pancreatitis (mild) is approached by allowing the amylase level to return to the normal level before performing LC. In this setting, many surgeons choose to perform a preoperative endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic sphincterotomy (ES) (if a stone is detected on ERCP) before LC. Bile duct stones will be found in only 10% to 15% of these patients, subjecting the remaining 85% to an unnecessary procedure and the risk of recurrent pancreatitis. Therefore, it is our practice to perform an intraoperative cholangiogram (IOC) at the time of LC and perform CBD exploration through the cystic duct or through a choledochotomy if stones are present. For a surgeon inexperienced in laparoscopic bile duct exploration, we advocate completion of the LC with postoperative ERCP and LS. Unless the stones are very large (greater than 1 cm), ERCP with LS has very high success rates and avoids the morbidity of an open cholecystectomy and bile duct exploration. However, conversion to open cholecystectomy with CBD exploration is a reasonable option, and should be chosen when the bile duct stones are large (>1 cm), when the CBD is extremely dilated (>12 mm), or the stone burden is formidable. Anatomic concerns, such as previous gastric bypass operation, will make ERCP a difficult option, favoring a direct approach to the CBD.
Cholangitis (jaundice, shaking chills, and right upper quadrant pain and tenderness) should be treated with preoperative ES and stone extraction unless the cholangitis is controlled easily with intravenous antibiotic therapy, as it often is. ERCP and ES should be successful at removing all CBD stones in more than 90% of patients with cholangitis. Laparoscopic bile duct exploration and stone extraction in this setting may be difficult because the stone is often impacted and the bile duct is inflamed and friable. If the cholangitis is cleared easily with antibiotics and the patient’s symptoms resolve, it may be appropriate to perform elective LC, operative cholangiography, and laparoscopic bile duct exploration if a stone is detected. Alternatively, LC can be performed shortly after the CBD has been cleared through ERCP and ES. The appropriate management of CBD stones in the era of LC remains fertile soil for good clinical studies.
Absolute contraindications to LC are few. Certainly, a patient who is unable to tolerate general anesthesia, a patient with gallbladder cancer (suspected or confirmed), and a patient with a “frozen upper abdomen” due to previous surgery or peritonitis should not undergo LC. In addition, relative contraindications include any previous right upper quadrant surgery, portal hypertension, coagulopathy, cholecystoenteric fistula, advanced acute cholecystitis, and pregnancy (during first and third trimesters). Although successful management of gallstone disease has been achieved in all of these patient groups, these procedures should be left for the surgeon who makes laparoscopy the majority of his or her practice and is technically skilled in these areas.
Preoperative Evaluation of A Patient with Gallstone Disease
In addition to a careful history and physical examination, preoperative testing for 90% of patients with gallstone disease is minimal. An ultrasound of the right upper quadrant usually confirms the diagnosis, and the hepatic biochemical profile (alkaline phosphatase, serum glutamic–oxaloacetic transaminase, bilirubin, and lactic acid dehydrogenase) usually determines the likelihood that CBD stones are present. Striking abnormalities in the hepatic profile or an ultrasonographically dilated CBD may warrant a preoperative ERCP or magnetic resonance cholangiography (MRCP), or both, to search for the cause (e.g., neoplasm or stricture) of the bile duct obstruction.
The diagnosis of biliary colic and acute cholecystitis, when the history and physical examination are less than definitive, can be made with a nuclear hepatobiliary hepatoiminodiacetic acid (HIDA) scan. An ejection fraction of less than 35% at 30 minutes suggests poor contractile function and incomplete emptying of the gallbladder. Removal of the gallbladder may benefit this patient population in the setting of cholelithiasis. Nonfilling of the gallbladder in 1 hour, especially after administration of intravenous morphine, which contracts the Oddi sphincter, is a highly accurate predictor of acute cholecystitis. When the ultrasound demonstrates nonuniform thickening of the gallbladder wall or pancreatic head, a computed tomographic scan should be performed to assess the likelihood of the presence of gallbladder cancer. Intravenous cholangiography, which is rarely performed in the United States, is popular in Europe for detecting CBD stones preoperatively in patients with abnormal liver function studies.
Patient Preparation, Equipment, and Anesthesia
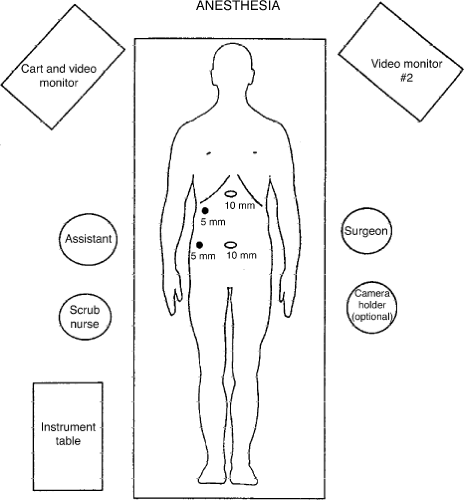
The first step in successful LC is the recruitment of a good operating team. This includes a well-trained laparoscopic surgeon, a first assistant with equivalent skills, and a camera operator who is familiar with the anatomy and technique of LC. With a well-choreographed operation, the first assistant can also be the camera operator. The elimination of a dedicated “camera operator” can only be achieved if the surgeon uses a two-handed operating technique. Ideally, the patient is placed on a fluoroscopic table with the table turned around backward for easy access to the upper abdomen by the C-arm. Two monitors are placed at the 10 o’clock and 2 o’clock position with respect to the patient’s head (Fig. 1). Generally, the surgeon stands to the patient’s left, and the first assistant stands to the patient’s right. If a dedicated camera operator is used, that person stands at the surgeon’s left.
Anesthetic techniques for laparoscopy are quite different than those used for open surgery. Generally, nitrous oxide is avoided to minimize the likelihood of bowel distention. Because insensible fluid losses through the closed abdomen are minimal, intravenous fluids should be run more judiciously than during open surgery. In addition, the pneumoperitoneum is a strong stimulus to antidiuretic hormone release, decreasing intraoperative urine output. If the anesthesiologist responds to oliguria with fluid boluses in the absence of fluid losses, pulmonary edema may result. Last, the anesthesiologist must be acutely attuned to ventilatory function, as the carbon dioxide pneumoperitoneum occasionally leads to hypercarbia and acidosis if close attention is not paid to the carbon dioxide concentration of expired gas (end-tidal PCO2). In addition, we ask our anesthesiologists to minimize the use of narcotics and administer a powerful antiemetic to lessen postoperative nausea. For elective LC where the gallbladder is not acutely inflamed, preoperative antibiotics have not been shown to be of benefit. All patients should have venous thromboembolic prophylaxis in the form of sequential compression devices prior to induction of anesthesia. Once the patient is anesthetized and intubated, a Foley catheter and an orogastric tube are generally placed. Decompression of the stomach and bladder minimizes the chance of injury during establishment of abdominal access.
The equipment for LC is quite simple. The only rigid requirements are that a high-quality videolaparoscope with a 300-W light source be coupled to two high-resolution monitors. A high-flow carbon dioxide insufflator, four trocars (two 10-mm trocars and two 5-mm trocars), and approximately 10 specialized laparoscopic hand instruments are required. We frequently use a monopolar electrode L-hook with suction and irrigation capacity, a fine-tipped dissector, two gallbladder graspers, a large gallbladder extractor, a pair of scissors, and a medium to large hemoclip applier. A 10-mm stone retrieval grasper is helpful to remove spilled gallstones. If a cholangiogram is to be performed, some surgeons prefer to use a small “microscissors,” a specialized cholangiogram clamp, and a 4 or 5 French tapered catheter. Techniques of cholangiography are discussed in a later section.
Pneumoperitoneum
LC is generally performed with a carbon dioxide pneumoperitoneum at 15 mm Hg pressure. There is no evidence that lower pressures confer a significant safety advantage but there is much interest in the use of alternative gases (e.g., nitrogen oxide, helium, and argon) for patients in whom excessive amounts of carbon dioxide may be detrimental. Generally, the pneumoperitoneum is obtained by sliding a specialized needle (a Veress needle) through the umbilicus, confirming its position by allowing saline to run through the needle from a plungerless syringe, and then attaching the needle to tubing from the carbon dioxide insufflator. Initially, the flow rate of carbon dioxide is kept below 2 L/min to ensure that proper placement has occurred before a large volume of gas is insufflated. Confirmation of the intra-abdominal position of the needle can be obtained by observing for uniform abdominal distention, tympani, and the ability to vary the intra-abdominal pressure by raising and lowering the abdominal wall. Initial pressures greater than 10 mm Hg nearly always reflect preperitoneal placement of the needle. Once the surgeon is comfortable that the needle is in the abdomen, the flow rate can be increased until an intra-abdominal pressure of 15 mm Hg is achieved.
The alternate method for establishing the pneumoperitoneum is to use an open Hasson cutdown technique, whereby the abdominal cavity is entered under direct vision. A scalpel, S-retractors, a clamp, and scissors are required for this technique. Once the peritoneal cavity is entered, the initial trocar is inserted and its position is secured with two stay sutures. The abdomen can then be insufflated rapidly with carbon dioxide. Other techniques used to obtain pneumoperitoneum include the opti-view port system in which the laparoscope is used in a specialized transparent port and is used to watch the trocar penetrate all layers of the abdominal wall. Any of these pneumoperitoneum techniques are acceptable but there appear to be fewer major vessel injuries when a novice or occasional laparoscopic surgeon uses an open access technique. Nevertheless, no technique is completely safe. Bowel and vascular injuries have been reported with all these techniques. Ultimately, the safest technique is the one that the surgeon is most comfortable performing.
Trocar Placement
Once the pneumoperitoneum has been established, the primary trocar is placed through the umbilicus. This will be a 10-mm trocar. Trocars are placed with a screwing, not a plunging, motion. It is generally a good idea to apply a second hand to the barrel of the trocar to prevent the inadvertent plunge that leads to intestinal or vascular injury. The assistant should provide countertraction on the abdominal wall during placement of the first trocar. If the patient is morbidly obese, a primary trocar position above the umbilicus allows better access to the right upper quadrant. If a previous abdominal surgery has been performed through a vertical midline incision, an alternate site for primary puncture is chosen. In this situation, we insufflate through a site adjacent to the umbilicus or in the left upper quadrant using a Veress needle technique, then place the primary trocar—a 5-mm trocar—in the right upper quadrant and pass a 5-mm telescope. The 10-mm umbilical trocar is then placed under direct vision, avoiding the adhesions from the previous operation. Many surgeons prefer to use the Hasson technique when previous abdominal surgery precludes primary puncture through the umbilicus. However, if the bowel is adherent to the undersurface of a previous midline incision, laparoscopy increases the risk of intestinal injury during the abdominal access maneuvers.
Once the umbilical trocar is established, a 10-mm telescope is passed through the primary trocar. An angled (30-degree) telescope allows a better view of the CBD, the posterior wall of the gallbladder, and the Calot triangle than does a straight (0-degree) telescope. Next, a 10-mm trocar is placed in the epigastrium, starting from the midline and angling toward the gallbladder. This trocar should enter at the level of the inferior liver edge just to the right of the falciform ligament. If this trocar is too low, the surgeon will be working parallel with his or her operating telescope. If this trocar is placed too high, segment IV of the liver will impede the surgeon’s ability to get to the gallbladder fossa. The third trocar is a 5-mm trocar, which is generally placed 2 to 3 cm below the costal margin in the midclavicular line. The fourth trocar is located in a variable position, generally in the anterior axillary line, several centimeters below the fundus of the gallbladder. If this port is placed too low, it will be impossible to lift the gallbladder up to expose the porta hepatis sufficiently (Fig. 2).
Exposure of the Porta Hepatis
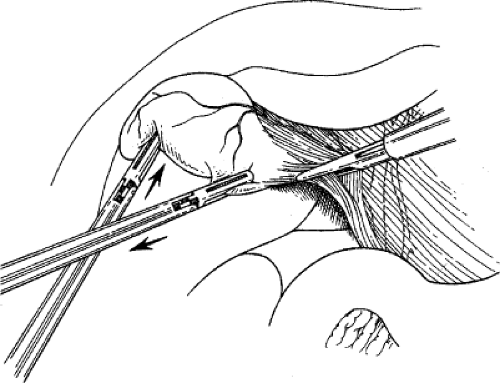
Exposure of the porta hepatis requires maximal elevation of the gallbladder fundus and liver edge. This elevation is usually achieved by the placement of a ratcheted, aggressive clamp on the fundus of the gallbladder from the most lateral trocar, and cephalad displacement is initiated until the infundibulum of the gallbladder, the duodenum, and the porta hepatis are well exposed (Fig. 2). If exposure of the porta hepatis is inadequate, the patient can be placed in a more severe reverse Trendelenburg position, the fundic grasper can be moved farther down the gallbladder to better elevate the gallbladder, or a fifth trocar can be introduced from the patient’s left side to push down on the duodenum. This last technique is rarely necessary.
Stripping the Peritoneum
Using a two-handed technique, the surgeon grasps the gallbladder infundibulum with an instrument in his or her left hand and
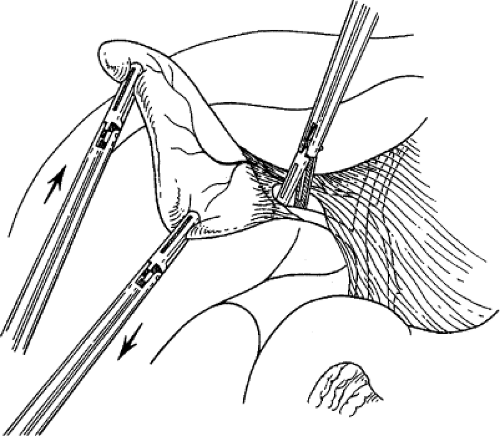
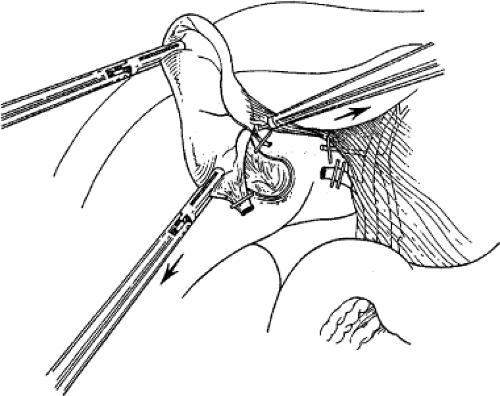
retracts it laterally. With a fine dissector, the peritoneum is torn at the interface between the gallbladder and periportal fat. The peritoneum is teased toward the common duct until the cystic duct, cystic artery, or lymph node of Calot is identifiable. Complete stripping of the posterior cystic duct is facilitated if the surgeon pushes the infundibulum medially to strip the peritoneum off the posterior aspect of the gallbladder and cystic duct. The 30-degree angled telescope is critical to obtaining this exposure. This lateral and medial retraction of the infundibulum is key for the safe circumferential visualization and dissection of the gallbladder infundibulum (Fig. 3).
retracts it laterally. With a fine dissector, the peritoneum is torn at the interface between the gallbladder and periportal fat. The peritoneum is teased toward the common duct until the cystic duct, cystic artery, or lymph node of Calot is identifiable. Complete stripping of the posterior cystic duct is facilitated if the surgeon pushes the infundibulum medially to strip the peritoneum off the posterior aspect of the gallbladder and cystic duct. The 30-degree angled telescope is critical to obtaining this exposure. This lateral and medial retraction of the infundibulum is key for the safe circumferential visualization and dissection of the gallbladder infundibulum (Fig. 3).
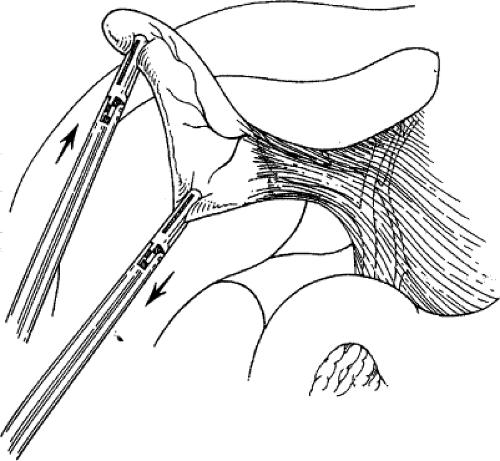 Fig. 3. The cystic duct is visualized by retracting the gallbladder infundibulum laterally and then stripping the peritoneum off the gallbladder. |
Pedunculation of the Gallbladder
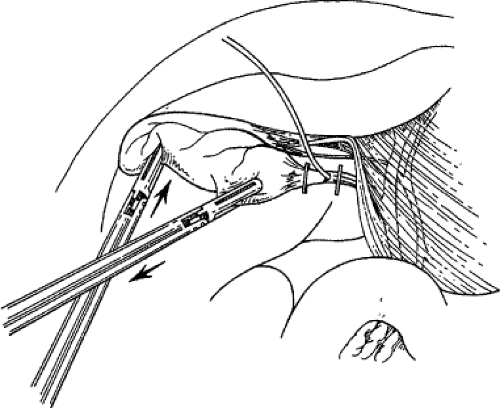
Stripping of the peritoneum over the gallbladder should reveal the presumed insertion of the cystic duct into the gallbladder. Continued dissection at this interface, first with a fine dissector and then with a monopolar L-hook between the cystic artery and cystic duct, provides the anatomic definition of important cystic duct anatomy. Complete dissection in this region allows the gallbladder to appear like a polyp on a stalk (the cystic duct). Careful dissection and division of the lymphatics that cross Calot’s triangle may facilitate the exposure of the cystic duct. The cystic artery that crosses Calot’s triangle may impede this pedunculation effort and may be divided between clips near the gallbladder, if necessary. Pedunculation of the gallbladder may be assisted by dividing the medial and lateral peritoneal attachments of the gallbladder to the liver along the body of the gallbladder. It is unnecessary to continue the dissection any farther down the cystic duct than is needed to place two clips on the structure. The CBD is usually seen with the angled scope, and it is almost never necessary to dissect the cystic duct down to its junction with the CBD. To do so is to risk injury to this structure (Fig. 4).
Control of the Cystic Duct and Cystic Artery
Generally, the cystic duct is narrow enough that an 8-mm (medium to large-sized) hemoclip can be passed around it and slid up to the infundibulum of the gallbladder, where it is closed. If cholangiography is to be performed (see Intraoperative Cholangiography), it is done now. If a cholangiogram is not to be performed, two clips are placed on the cystic duct immediately below its junction with the gallbladder, and the cystic duct is divided. A long cystic duct remnant is not a concern as long as no stones are retained in this remnant. Two hemoclips are placed on the cystic artery as it crosses onto the gallbladder, if it has not been previously clipped, and the cystic artery is divided (Fig. 5).
Resection of the Gallbladder
If adequate pedunculation has been performed before cystic duct division, the gallbladder should already be dissected off the liver a quarter of the way to the fundus. Gallbladder resection is facilitated by strong use of the retracting (left) hand to pull the gallbladder away from the liver. As the gallbladder is pulled away from the liver, the monopolar electrode is used to coagulate the small bridging veins and areolar tissue connecting the gallbladder to the liver (Fig. 6). If hemorrhage occurs during this dissection, it usually means that the surgeon is not in the right tissue plane, most frequently in the hepatic parenchyma. Holes in the gallbladder occur frequently during this dissection. They can usually be controlled with a pretied chromic ligature, an interrupted figure-eight suture, or carefully placed hemoclips. When the fundus of the gallbladder is reached, the majority of the gallbladder is flipped over onto the anterior surface of the liver and used as a handle to retract the gallbladder
fossa, and hemostasis of the gallbladder fossa is checked (Fig. 7). The remaining peritoneum connecting the gallbladder and liver is then divided with electrosurgery to disconnect the gallbladder from the liver.
fossa, and hemostasis of the gallbladder fossa is checked (Fig. 7). The remaining peritoneum connecting the gallbladder and liver is then divided with electrosurgery to disconnect the gallbladder from the liver.
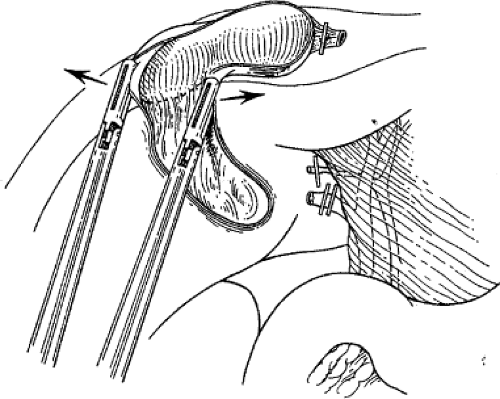 Fig. 7. Excellent exposure of the gallbladder bed and remaining peritoneum is gained by “flipping” the gallbladder over the liver edge and stretching apart the last bit of peritoneum with two graspers. The arrows indicate the direction of retraction of the gallbladder for optimal exposure.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|