The expiration date of the reagent should be readily apparent on the container. For commercially procured reagents, the manufacturer-assigned expiration date is often used. If no expiration date is available, and the material is stable, a company may have a procedure in place that would allow it to assign a predefined expiration period based on the date of receipt. Shorter expiration dates may be required if stability is an issue for the material. For example, solutions that are prone to microbial growth, such as high-performance liquid chromatograph (HPLC) water, an opened date and use-by date may be added to the container upon opening. Table 24.1 contains some typical expiry dating periods for common solutions.
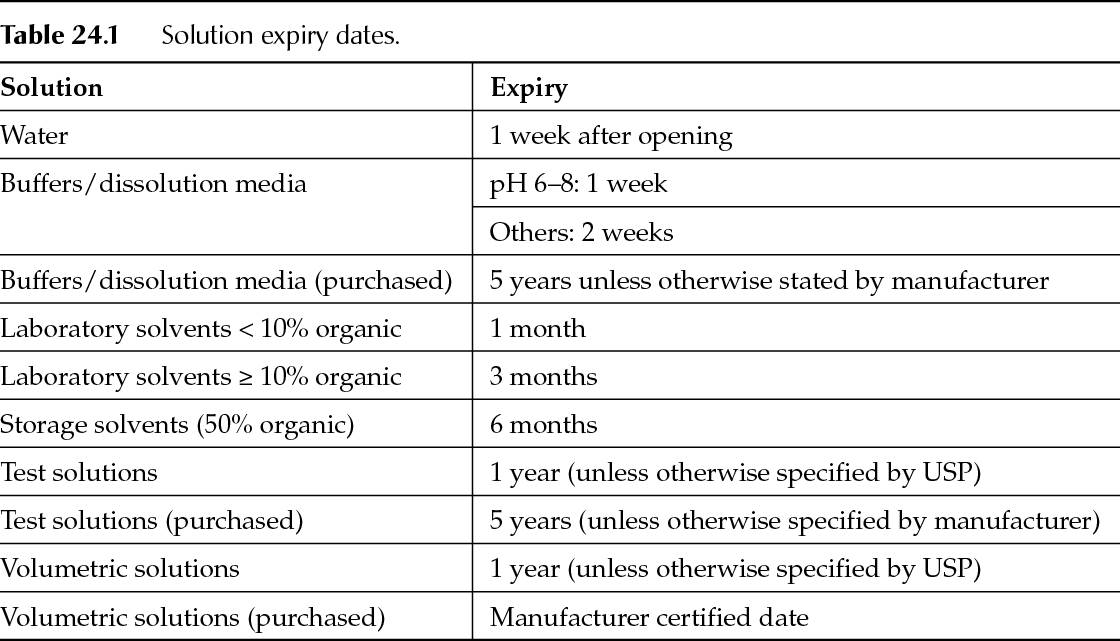
Some reagents and solutions may be used past their expiration date if the material can be recertified or re-qualified. For example, it is common to re-standardize or recertify volumetric solution upon use or at monthly intervals. The current compendia (European Pharmacopeia [PhEur], Japanese Pharmacopeia [JP], and United States Pharmacopeia [USP]) typically define the process for re-standardizing the most commonly used volumetric solutions.
The preparation of all solutions prepared in the laboratory must be documented. The prepared solution should be identified with, at a minimum, the name/composition of the solution, concentration, storage, re-standardization date, expiration date, reference, and analyst/date.
Standards
Laboratories use primary and secondary standards to determine material results and equipment calibration. A primary standard is purchased from an official source, such as USP, British Pharmacopeia (BP), or National Institute of Standards and Technology (NIST). If no official source of a standard exists, material can be thoroughly characterized to ensure its identity, strength, quality, purity, and potency. Secondary or in-house standards are raw materials that have been assayed against a primary standard.
When standards are received into a laboratory, traceability must be established. Most laboratories will assign a unique standard number and complete corresponding paperwork indicating the standard’s name, lot number, purity, expiration date, and drying requirements. USP has a bimonthly publication that reports expiration dates. Analysts must verify they are not using expired standards. Based on the USP publication, most companies will establish a bimonthly review of their standards to ensure no expired ones are in the laboratory.
Sample Storage
The storage of samples is extremely important to ensure the integrity of the material. Once sample containers are received into the laboratory, the containers should be stored in a centralized location until testing is started. Samples should have some form of unique identification to differentiate them from the other samples in the laboratory. Once testing has been started, procedures will dictate how samples are prepared and stored. For example, if a sample is light sensitive, amber glassware is used in the sample preparations. During method validation, solution stability is determined. Upon completion of testing, most laboratories require solutions to be retained, even if the solution has expired, until data review is complete. If there is a need for an investigation, the expired solutions may be used for information.
21 CFR 211.170 requires a reserve sample that is representative of the batch to be retained. The reserve sample consists of at least twice the quantity necessary to conduct all required testing. Reserve samples are typically pulled during inspection sampling and/or final packaging. The reserve samples are maintained at warehouse conditions and evaluated once a year for signs of deterioration. The finished product reserve samples are maintained for one year past their expiration date. The raw material reserve samples must be maintained one year past the expiration of the last finished product they were used in. Most pharmaceutical companies have finished products with a maximum of 36-month expiration dating; thus, they can assign a five-year expiration date to the raw material reserve sample, which ensures they will meet the CFR requirement.
Reagents or Solutions
Chemicals, reagents, and solutions must be stored in the laboratory in a manner that considers safety, chemical integrity, and efficient management. Materials must be stored in tightly sealed and chemically resistant containers. Strong bases should not be stored in glass containers because the hydroxide will slowly react with the silica. Exposure of materials to heat or direct sunlight should be avoided. If a material is easily oxidized, it may be stored with a layer of nitrogen. Some companies store those chemicals in desiccators and flush with nitrogen. Materials requiring refrigeration or freezing should be stored as such. Chemically incompatible materials will be segregated according to their MSDS requirements. Acids and bases are not stored together.
Standards
Standards are stored according to the label instructions. Typically, standards are maintained in desiccators until use. If drying is required before use, the analyst should take only the amount needed and dry in an appropriate container. Once dry, the analyst can store the dried standard in a desiccator until it is needed. Unless otherwise specified, standards are typically weighed and diluted within 24 hours of removal from the oven. After weighing, the remaining dried material is discarded. The appropriate storage of the standard solution should be outlined in the company’s method. During method validation, standard solution stability should be determined.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


