Summary by Elizabeth A. Warner, MD
19
Based on “Principles of Addiction Medicine“ Chapter by Elizabeth A. Warner, MD, and Emily Lorch, MD
APPROACH TO DRUG TESTING
Commonly used body fluids for drug testing include urine and blood. Urine is the most common source for testing for drugs of abuse; but its use is limited by adulteration or substitution of specimens. Urine specimen validity testing for urine creatinine, pH, specific gravity, temperature, and the presence of adulterants is required in federal workplace testing, but not in clinical settings. Blood testing is more accurate than urine testing for quantifying recent ingestion. Proper collection and processing of specimens are essential for accurate results. The timing of the collection should be noted, so results can be interpreted in the context of the time of ingestion. Chain-of-custody regulations in forensic or workplace testing have been developed to prevent specimen misidentification but are not routinely performed in clinical testing.
The initial procedure for drug testing usually is a screening urine immunoassay. Laboratories often offer panels that include multiple tests for commonly used drugs. Immunoassays are inexpensive, are easily automated, and yield rapid results. In these tests, an antibody is designed to detect a specific target, which may be a specific class of drug, a parent drug, or a metabolite. A major limitation of immunoassays is cross-reactivity. The antibody can interact with drugs other than the targeted substance, yielding a false-positive result.
Confirmatory testing, using gas or liquid chromatography followed by either mass spectroscopy (MS) or tandem mass spectroscopy (MS/MS), can more precisely identify drugs of abuse but is often not available on-site in hospital or office laboratories. Because of the delay associated with using an outside laboratory, results of confirmatory studies are not likely be available for clinical decision making in urgent clinical settings.
A cutoff is a defined concentration of an analyte in a specimen at or above which the test result is reported as positive and below which it is reported negative. If a drug is detected in a specimen in concentrations lower than the defined cutoff, the test result will be reported as negative, even though the drug was in fact detected. Knowledge of the cutoff level for a particular test is helpful in estimating the length of time after drug ingestion that a test result will be positive. Lower cutoff levels are associated with a higher sensitivity and with longer detection times, but also cause more false-positive results. Table 19-1 lists approximate duration of detection time from the time of last use, using commonly used cutoffs.
TABLE 19-1. APPROXIMATE DETECTION TIME USING SCREENING URINE IMMUNOASSAYS (WITH COMMONLY USED “CUT-OFFS”)
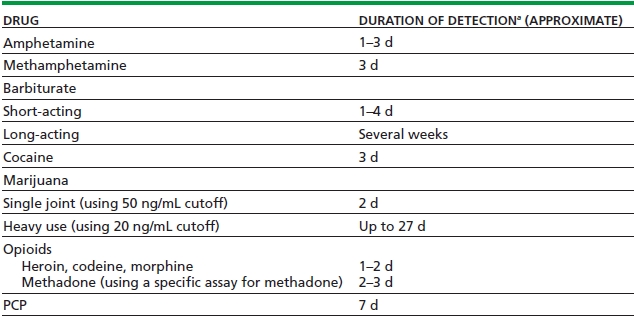
aThe duration of detection is variable and depends on dose, route of administration, pattern of use, laboratory cutoff, and individual metabolism.
The federal guidelines for federal workplace and DOT drug testing limit drug testing to five substances (amphetamines, cannabinoids, cocaine, opiates, and PCP) and establish cutoff values for screening and confirmatory testing of these substances.
Clinical laboratories perform drug and alcohol testing for diagnostic purposes and can select which substances to include in their drug screens, and the assays may vary among laboratories. Specimens obtained for clinical use, however, are not subject to the same collection and testing requirements and are not required to use the same cutoffs, as in federal workplace testing.
Point-of-care or on-site testing refers to tests that are performed outside of a central laboratory. Commercially available immunoassay kits are available to test urine or oral fluid for commonly abused drugs. The interpretation of these tests can be subjective, making them operator dependent. Most studies find that point-of-care testing is fairly reliable, with results comparable to automated immunoassays. As these point-of-care tests are designed for use as screening tests, many manufacturers recommend that any positive results be confirmed by more specific laboratory methods.
DRUG-SPECIFIC TESTS
Alcohol
Laboratory testing can be done to confirm recent alcohol intake but does not necessarily determine impairment, because some individuals develop tolerance to the effects of alcohol. Blood alcohol concentration detects alcohol use within the preceding few hours. Most states define intoxication based on whole-blood ethanol levels; common cutoffs are 80 or 100 mg/dL. Clinical laboratories, however, measure ethanol in serum or plasma. Because the water content of serum is higher than that of whole blood, with an estimated ratio of serum to whole blood of 1.14/1.00, the same specimen will have a higher level of ethanol in serum than in whole blood.
Less invasive means of detecting the blood alcohol concentration include analysis of alcohol in the exhaled air. Breath alcohol testing is usually done in traffic law enforcement and DOT testing. The ratio of blood to breath concentration of alcohol is approximately 2,100:1. In the United States, breath alcohol concentration is usually reported in grams per 210 L, so that a breath level of 0.1 g/210 L is equivalent to a whole-blood alcohol level of 100 mg/dL.
While measurements of alcohol concentration may help determine recent ingestion, other blood alcohol biomarkers are used to predict alcohol-related health problems. Blood tests that are considered markers for alcohol use disorders include gamma-glutamyl-transferase (GGT), aspartate amino transferase (AST), and erythrocyte mean cell volume (MCV). Of these tests, the GGT is the most sensitive marker of alcohol abuse. GGT levels are elevated in approximately 75% of persons with diagnosed alcohol dependence, approximately 50% of patients hospitalized for alcohol-related problems, and approximately 30% of people who drink heavy amounts and have related problems. It is important to note that the GGT is not specific for alcohol use disorders and is also elevated in patients with fatty liver and obstructive liver disease and in those using certain medications, including anticonvulsants.
The MCV (mean corpuscular volume), a measurement of red blood cell size, increases with chronic heaving drinking. With abstinence, the MCV will fall, but because the life span of the red blood cell is 120 days, it may take approximately 3 months to see improvement in the MCV after abstinence. Serum aminotransferases, particularly AST and alanine amino transferase (ALT), may be elevated in patients with alcohol use disorders. However, these tests are not as sensitive as markers for alcohol abuse as the GGT. The AST is usually more elevated than the ALT in patients with alcohol-related liver disease. When the aminotransferases are elevated and ALT is more elevated than the AST, alcohol is less likely to be the cause of the liver disease. Although elevations of the aminotransferases may suggest alcohol-related liver disease, none of the abnormal liver enzymes can predict alcohol dependence or intoxication.
Amphetamines
Amphetamines are a group of stimulants that include amphetamine, methamphetamine, “Ecstasy,” which is MDMA (3,4-methylenedioxymethamphetamine) or MDA (3,4-methylenedioxyamphetamine), and “Eve,” which is MDEA (3,4-methylenedioxy-N-ethylamphetamine). Amphetamine is a metabolite of methamphetamine. Both amphetamine and methamphetamine have d– and l-isomers. As the d-isomers of amphetamine and methamphetamine have stronger central nervous system effects, they are associated with more abuse potential than the l-isomers. Screening tests for amphetamines are usually targeted to methamphetamine and can have variable cross-reactivity with MDMA, MDEA, and MDA. Routine GC/MS amphetamine assays can distinguish methamphetamine from amphetamine but cannot distinguish the d-isomers from the l-isomers.
Of the frequently tested drugs of abuse, amphetamines have the most false-positive screening tests because of cross-reactivity on immunoassay with many sympathomimetic amines. Examples of drugs potentially causing false-positive results include phenylpropanolamine, pseudoephedrine, and l-methamphetamine and appetite suppressants containing ephedrine and phentermine. Other prescription drugs, such as selegiline or benzphetamine, are metabolized either to amphetamine or to methamphetamine and can also cause positive results. While Adderal (mixture of l-amphetamine and d-amphetamine) will give a positive test for amphetamine, Ritalin (methylphenidate) is not metabolized to amphetamine or methamphetamine and is not detected on confirmatory testing for amphetamine or methamphetamine.
Barbiturates
Barbiturates, which are central nervous system depressants, are classified by their duration of action. Most urine immunoassays are directed toward secobarbital, a short-acting barbiturate. The duration of detection in the urine after barbiturate use is variable and depends on dose. In general, short-acting barbiturates such as butalbital, pentobarbital, and secobarbital, can be detected from 1 to 4 days after use, while long-acting barbiturates such as phenobarbital can be detected for several weeks after use.
Benzodiazepines
The interpretation of urine immunoassays for benzodiazepines is complicated by the multiple drugs available, their variable potencies (allowing a large dose range), and their diverse metabolites. Urine specimens usually contact little of the parent benzodiazepine. Many benzodiazepines show poor cross-reactivity with commonly used immunoassays, which can be explained by their metabolic pathways. For example, chlorazepate, chlordiazepoxide, and diazepam are metabolized to nordiazepam and oxazepam. Clonazepam is metabolized to 7-aminoclonazepam. Alprazolam, lorazepam, and triazolam are excreted as glucuronide conjugates, distinct from oxazepam conjugates. Since many of the benzodiazepine immunoassays are commonly designed to detect nordiazepam or oxazepam, they are less likely to detect clonazepam, lorazepam, or triazolam, unless present in high concentration. Neither the benzodiazepine antagonist, flumazenil, nor zolpidem or eszopiclone are detected by benzodiazepine assays.
Cocaine
Cocaine hydrochloride is the powdered form of cocaine. It is water soluble and can be snorted or mixed with water and injected. The alkaloid form of cocaine, “crack” or freebase, is not water soluble but vaporizes when heated and can be smoked. Screening urine immunoassays measure benzoylecgonine, the major urinary cocaine metabolite, commonly using a cutoff of 300 ng/mL. The detection of cocaine in the urine is variable and depends on the amount of drug ingested. The usual detection time after cocaine use is 2 to 3 days, though there are reports of positive urine assays up to 22 days after high-dose binges. Immunoassays for benzoylecgonine are quite specific and have not been reported to have false positives with other drugs.
Marijuana
The primary psychoactive component of the marijuana plant is tetrahydrocannabinol (THC). When smoked, THC is absorbed quickly into the circulation, with an elimination half-life estimated to be between 20 and 30 hours. THC has a highly lipophilic nature and is stored in fat tissues, where it is slowly released back into the circulation. Most laboratories measure the inactive metabolite 11-nor-Δ9-THC-9-carboxylic acid (THC-COOH).
Urine screening tests for marijuana typically use cutoffs of 20, 50, or 100 ng/mL. The current federally mandated cutoff for workplace testing is 50 ng/mL. The detection time for marijuana is variable and depends on the amount ingested, whether the person is a chronic or an occasional user, and the sensitivity of the assay. The mean detection time of a single marijuana cigarette is <2 days using a 50 ng/mL cutoff immunoassay. In frequent users, urine specimens were found positive for THC-COOH up to 27 days after last use, with an immunoassay with a cutoff of 20 ng/mL. A positive test can identify recent marijuana use, but it does not correlate with level of impairment. Oral ingestion of hemp seed oil or dronabinol can result in positive urine test results for marijuana.
Opiates and Opioids
Opiates are drugs derived from opium, the extract of the seeds of the opium poppy, and include morphine, codeine, and heroin. The term opioid is more comprehensive and includes all agonists and antagonists with morphine-like activity, including natural opiates, semisynthetic drugs such as hydrocodone and hydromorphone, and synthetic drugs, such as oxycodone, methadone, buprenorphine, and fentanyl. The evaluation of opiate drug screens can be confusing. Many physicians assume the immunoassays for “opiates” are a reliable screen for all opioids. However, currently available opiate immunoassays are targeted to detect opiates, using morphine for the target. These opiate immunoassays have little or no cross-reactivity with synthetic opioids such as fentanyl, oxymorphone, meperidine, propoxyphene, buprenorphine, or methadone and variable cross-reactivity with hydrocodone, hydromorphone, and oxycodone. For example, a patient taking oxycodone can have a negative urine drug screen for opiates. To help identify the presence or absence of specific synthetic opioids, immunoassays designed to detect oxycodone, fentanyl, methadone, or buprenorphine are available.
Knowledge of the metabolic pathways of commonly prescribed opioids is required to accurately interpret opiate screening and confirmatory testing. The standard opiate screen is targeted to morphine, so any compounds that are metabolized to morphine are likely to be detectable on a screening opiate test. This includes heroin, codeine, morphine, or poppy seeds (which contain small quantities of codeine and morphine). In the process of heroin metabolism, a short-acting metabolite, 6-monoacetylmorphine (6-MAM), is produced, which then is hydrolyzed to morphine. The only finding in the urine that unequivocally proves heroin use is the detection of 6-monoacetylmorphine. This metabolite is the specific by-product of heroin metabolism and not a metabolite of morphine, codeine, poppy seeds, or other synthetic opioids. However, 6-MAM is rapidly eliminated and usually detected in the urine for <8 hours after heroin use. Although regulations for federal workplace programs require testing for 6-MAM, it is not routinely included in clinical testing.
The interpretation of confirmatory testing for specific opioids is complicated by the fact that some prescribed opioids are actually minor metabolites of other opioids (Table 19-2). For example, hydrocodone is minor metabolite of codeine, oxymorphone is a metabolite of oxycodone, and hydromorphone is a metabolite of hydrocodone and morphine. Therefore, GC/MS may detect oxymorphone in a patient who is prescribed oxycodone, as oxymorphone is a minor metabolite of oxycodone.
TABLE 19-2. MONITORING OPIOID THERAPY: EXPECTED RESULTS ON URINE OPIATE SCREEN AND CONFIRMATORY TESTING
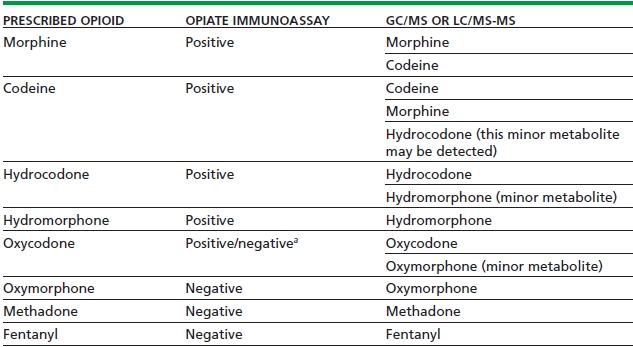
aDepends on the cross-reactivity of the opiate assay with the prescribed drug; varies among assays.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


