Table 20-1. Drug-Induced Causes of AKI
Type of AKI | Causative drugsa |
Prerenal AKI | ACEIs, ARBs, COX-2 inhibitors, cyclosporine, diuretics, NSAIDs, radiocontrast media, renin inhibitors, tacrolimus |
Intrinsic AKI |
|
Vascular | Vasculitis and thrombosis: Bevacizumab, cisplatin, cyclosporine, hydralazine, methamphetamines, mitomycin C, propylthiouracil, tacrolimus |
| Cholesterol emboli: Warfarin, thrombolytic agents |
Glomerular | COX-2 inhibitors, gold, heroin, lithium, NSAIDs, pamidronate, phenytoin |
Interstitial nephritis | Allergic interstitial nephritis: Ciprofloxacin, COX-2 inhibitors, NSAIDs, penicillins, proton pump inhibitors |
| Chronic interstitial nephritis: Aristolochic acid (Chinese herbs), cyclosporine, lithium |
| Papillary necrosis: Analgesic combinations |
Tubular epithelial cell damage | Acute tubular necrosis: Adefovir, aminoglycosides, amphotericin B, carboplatin, cidofovir, cisplatin, cocaine, cyclosporine, foscarnet, ifosfamide, radiocontrast media, tacrolimus, tenofovir, zoledronate |
| Osmotic nephrosis: Dextran, hydroxyethyl starch solutions, mannitol, sucrose-containing intravenous immunoglobulin |
|
|
Postrenal AKI |
|
Obstructive | Acyclovir, foscarnet, indinavir, methotrexate, oxalate, sulfonamides |
Nephrolithiasis | Allopurinol, indinavir, sulfonamides, triamterene |
Nephrocalcinosis | Oral sodium phosphate solution |
a. This list does not include all potential nephrotoxins.
■ Tubular—accounts for 90% of intrinsic cases: Intrarenal vasoconstriction, direct tubular toxicity, and intratubular obstruction; prolonged ischemia from prerenal causes; and toxins that may be endogenous or exogenous
• Endogenous: Myoglobin, hemoglobin, and uric acid
• Exogenous: Medications (see Table 20-1; aminoglycosides are common nephrotoxins leading to nonoliguric acute tubular necrosis after 5–7 days of therapy) and other exogenous substances such as ethylene glycol and pesticides
Postrenal AKI
This disease is an obstruction of urinary flow at any level from the urinary collecting system to the urethra. It must involve both kidneys (or one kidney in a patient with a single functioning kidney). Medications associated with postrenal AKI are identified in Table 20-1.
Etiologies by anatomic site are as follows:
■ Renal pelves or tubules: Crystal deposition
■ Ureteral: Tumor, stricture, and stones
■ Bladder neck obstruction: Prostatic hypertrophy and bladder carcinoma
Diagnostic Criteria
Table 20-2 shows the diagnostic tests and the findings associated with AKI. A diagnosis requires the following:
■ Evaluate physical findings: Assess for signs and symptoms listed in clinical presentation.
■ Take medication history (including over-the-counter medication and herbals): Identify potentially nephrotoxic agents (Table 20-1). Note: Patients with contrast-induced nephrotoxicity (CIN) may have received the dose 24–72 hours before the time when the rise in serum creatinine is noted.
■ Estimate GFR: Normal is generally in the range of 90–125 mL/min/1.73 m2.
• Consider limitations in using serum creatinine as a marker of kidney function (e.g., conditions of poor muscle mass) and in using equations to estimate GFR in patients with unstable kidney function. See discussion of assessment of kidney function in Section 20-4.
• Other assessment equations and methods (e.g., Jelliffe and Jelliffe equation) are available to estimate GFR in patients with unstable kidney function.
Blood tests
■ Elevated: Serum creatinine, blood urea nitrogen (BUN), and electrolytes (potassium and phosphorus). The BUN/serum creatinine ratio may be elevated in prerenal AKI.
■ Decreased: Calcium (consider albumin concentration to correct calcium), bicarbonate
Urinalysis
■ A low urine sodium and an elevated specific gravity and osmolality are indicative of prerenal causes and stimulation of sodium and water retention.
Table 20-2. Laboratory Findings to Differentiate Prerenal and Intrinsic AKI

BUN, blood urea nitrogen; Cr, creatinine; FENa, fractional excretion of sodium; RBC, red blood cell; WBC, white blood cell.
■ Proteinuria includes the following:
• Albuminuria: Microalbuminuria or moderately increased albuminuria (> 30 mg/day) and severely increased albuminuria (> 300 mg/day)
• Proteinuria (includes albumin and other proteins): Moderately increased (> 150 mg/day) and severely increased (> 500 mg/day) proteinuria and nephrotic range proteinuria > 3.5 g/day
■ Hematuria is indicated by red blood cells.
■ Glucose and ketones may be present.
■ Urine sediment consisting of granular casts and cellular debris suggests structural damage (hyaline casts are normal).
■ White blood cells suggest inflammation.
■ Eosinophils are associated with acute allergic interstitial nephritis.
■ Consider whether fluids or diuretics were previously administered when interpreting urinalysis.
Urine chemistries
Evaluate urine sodium, potassium, chloride, creatinine, and urinary anion gap.
Fractional excretion of sodium (FENa) is useful to differentiate prerenal AKI from intrinsic AKI. A low value (< 1%) suggests retention of sodium and water (prerenal etiology) versus intrinsic cause.
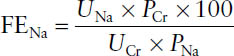
where
UNa = urine sodium
UCr = urine creatinine
PCr = plasma creatinine
PNa = plasma sodium
Other tests
Radiographic procedures include ultrasound, plain film radiograph, radioisotope scan, and computed tomography.
Renal biopsy may be indicated for patients without cause of AKI identified by other diagnostic tests.
Treatment Principles and Goals
Prevention
Identify risk factors:
■ Volume depletion
■ Exposure to nephrotoxic medications
■ Preexisting kidney or hepatic disease
Surgical procedures can be a risk factor. Consider baseline kidney function, age, cardiovascular status, and volume status.
Diagnostic tests requiring radiocontrast media can put patients at risk of AKI. Contrast agents are hyperosmolar compared to plasma osmolality and may cause osmotic diuresis, dehydration, and renal ischemia. Risk factors for CIN include diabetes, heart failure, age > 75, hypotension, and an estimated GFR < 60 mL/min/1.73 m2. In addition to being well hydrated, usually with intravenous (IV) fluid administration, high-risk patients may benefit from oral acetylcysteine (Mucomyst) given as 4 doses of 600 mg or 1,200 mg every 12 hours with the first dose given before exposure to radiocontrast dye. Of note, in a prospective study in patients undergoing coronary and peripheral vascular angiography, acetylcysteine did not reduce the risk of CIN; therefore, this strategy is not consistently supported by available evidence. Bicarbonate may also be added to the hydration fluid.
Treatment goals
■ Correct underlying causes of AKI (e.g., discontinue nephrotoxic agents, correct fluid status, treat underlying infection, address cause of urinary tract obstructions).
■ Return to baseline kidney function or highest kidney function possible.
■ Prevent development of chronic kidney disease and the need for chronic renal replacement therapy.
■ Avoid nephrotoxic agents or take measures to reduce exposure if possible.
■ Adjust doses of medications on the basis of kidney function. As kidney function recovers drug doses may need to be increased.
■ Avoid agents contraindicated in patients with kidney disease, such as metformin (Glucophage) and gadolinium-based contrast dyes used for magnetic resonance imaging (MRI) procedures. Gadolinium has been shown to cause nephrogenic systemic fibrosis (NSF) in patients with preexisting kidney disease. NSF is a fibrosing disorder that involves predominantly the skin but also affects systemic organs such as the liver, heart, lungs, diaphragm, skeletal muscle, and joints.
■ Address complications of AKI, such as electrolyte abnormalities (hyperkalemia), fluid overload, metabolic acidosis (see Chapter 21 on fluids and electrolytes), and hyperphosphatemia (see Section 20-4 on chronic kidney disease).
Strategies for treatment
■ Address underlying cause of AKI.
■ Provide supportive care with diuretic therapy (loop diuretics) and replacement fluids as needed to maintain hemodynamic stability.
Drug Therapy
Diuretics
Loop diuretics are recommended for management of fluid overload in patients with AKI (Table 20-3). They are not advocated to prevent or treat AKI and have not been shown to significantly improve outcomes of AKI.
Mechanism of action
Loop diuretics are delivered to the tubular lumen of the kidney by active secretion from the blood into the urine at the proximal tubule. Binding of the diuretic to albumin allows the drug to be confined to the plasma and minimizes glomerular filtration so the diuretic may be delivered to the organic acid secretory sites and transported into the lumen. Once at the site of action, loop diuretics cause inhibition of sodium and chloride reabsorption in the thick ascending limb of the loop of Henle to promote water excretion.
Thiazide and thiazide-like diuretics inhibit the Na+-Cl− cotransport in the early distal convoluted tubules. They are generally used in combination with loop diuretics for resistant edema and fluid overload, particularly metolazone (Zaroxolyn, Mykrox), which is effective at GFR < 30 mL/min. Other thiazide diuretics are generally not effective when GFR is < 30 mL/min.
Adverse drug events
Loop diuretics
Adverse drug events are as follows:
■ Hypokalemia, hypomagnesemia, hyponatremia, hypovolemia, hyperuricemia, hyperglycemia
■ Hypercalciuria, hypocalcemia
■ Orthostatic hypotension, dehydration
■ Metabolic alkalosis (partly attributable to extracellular fluid volume contraction)
■ Ototoxicity
■ Diarrhea, nausea
Furosemide (Lasix), bumetanide (Bumex), and torsemide (Demadex) have a sulfonamide substituent (potential for hypersensitivity reactions). Ethacrynic acid (Edecrin) is generally reserved for patients allergic to sulfa compounds.
Thiazide and thiazide-like diuretics
Adverse drug events include the following:
■ Hypokalemia, hyponatremia, hypercalcemia, hyperuricemia
■ Hypovolemia, orthostatic hypotension
■ Hyperglycemia, hypochloremic alkalosis, hyperlipidemia
■ Hypersensitivity reactions from sulfonamide substituents
■ Chest pain (metolazone; more common with Mykrox, which is more rapidly and extensively absorbed than Zaroxolyn)
Drug–drug and drug–disease interactions
■ Loop diuretics and aminoglycosides have an increased potential for ototoxicity.
■ Diuretics and other nephrotoxins have an increased risk of nephrotoxicity if hypovolemia occurs.
Table 20-3. Loop Diuretics for Fluid Overload in AKI
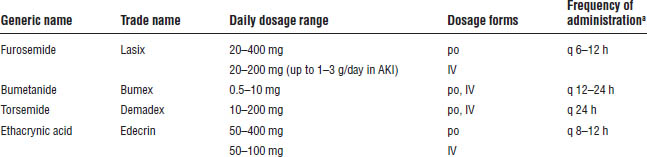
a. Loop diuretics are also administered as a continuous infusion. Higher dose ranges for intermittent dosing are reserved for patients who are unresponsive to initial smaller doses.
■ Diuretics and lithium (Lithane, Lithobid) used concomitantly may result in decreased renal clearance of lithium. Monitor lithium concentrations more closely.
■ For diuretics and digoxin (Lanoxin, Digox), hypokalemia from diuretic use may increase risk of toxicity with digoxin. Monitor potassium and digoxin.
■ Loop and thiazide diuretics may increase gout attacks because of hyperuricemia.
■ For thiazide diuretics and diabetes, hyperglycemia may result from thiazides. Increase glucose monitoring.
■ The following conditions decrease secretion of the diuretic to its site of action in the renal tubule:
• Proteinuria (diuretic binds to protein and is not available at its site of action)
• Decreased renal blood flow
• Competitive inhibition of transport system (NSAIDs, probenecid [Probalan], cephalosporins)
Parameters to monitor
■ Blood pressure (sitting and standing), pulse, urine output, fluid intake, serum creatinine, serum electrolytes, BUN, bicarbonate, calcium, glucose, uric acid
Pharmacokinetics
Loop diuretics
■ Oral bioavailability: Furosemide (60%), bumetanide (85%), torsemide (85%)
■ Oral:intravenous dose ratios: Furosemide (1.5), bumetanide (1), torsemide (1)
■ Equivalent doses: 1 mg bumetanide = 20 mg torsemide = 40 mg furosemide
■ Elimination route: Furosemide (primarily renal), bumetanide (hepatic and renal), torsemide (primarily hepatic), ethacrynic acid (hepatic and renal)
Thiazide and thiazide-like diuretics
Metolazone absorption differs between brands. Mykrox (available outside the United States) is more rapidly and extensively absorbed than Zaroxolyn.
Other factors
Patients with kidney disease generally require larger doses of diuretics to achieve adequate concentrations of the drug at the site of action in the kidney.
The brands of metolazone (Zaroxolyn and Mykrox) are not bioequivalent and should not be interchanged.
Dopamine
Low-dose dopamine is a potent vasodilator that increases renal blood flow and has been associated with an increase in urine output in AKI. Most clinical studies, however, have not shown improvement in recovery from AKI or mortality rates. KDIGO guidelines do not advocate dopamine for AKI.
Nondrug Therapy
Fluid management
Fluid intake and output should be evaluated and adjustments made to maintain hemodynamic stability (consider sensible and insensible losses).
Fluid selection (e.g., crystalloids, colloids, or normal saline) and rate of correction depend on the clinical condition of the patient. Note: Hetastarch should not be used in critically ill patients because of the increased risk of mortality and renal injury requiring renal replacement therapy in certain populations (e.g., patients in the intensive care unit, septic patients, patients with preexisting kidney dysfunction).
Nutritional therapy
A high-calorie diet is generally required (patient specific).
Restriction of sodium, potassium, and phosphorus should be considered.
Renal replacement therapies
Renal replacement therapies are procedures by which the blood is artificially cleared of waste and some essential metabolic products to augment the function of failed or failing kidneys. These procedures include hemodialysis and hemofiltration, in which the semipermeable membrane is a dialyzer, and peritoneal dialysis, in which the peritoneal cavity serves as this membrane. Procedures may be intermittent or continuous. Hemodialysis and hemofiltration are the modalities used for patients with AKI. Continuous renal replacement therapies (CRRTs) require lower blood and dialysate flow rates and remove fluid more gradually than intermittent procedures and are used often for patients with AKI who are hemodynamically unstable. Common terminologies used for CRRT include continuous venovenous hemofiltration, continuous venovenous hemodialysis, and continuous venovenous hemodiafiltration.
The potential for drug removal by dialysis must be considered, particularly for CRRT procedures that are performed over a prolonged period of time (e.g., > 24 hrs). Drug doses may need to be increased to account for removal by CRRT (e.g., certain antibiotics). Drug characteristics that make a drug more likely to be removed by dialysis are small molecular weight, low protein binding, and low volume of distribution.
Indications for renal replacement therapy
Any of the following refractory to more conservative measures is an indication for renal replacement therapy:
■ Acidosis
■ Electrolyte abnormalities (hyperkalemia)
■ Intoxication (drug-induced kidney failure), if drug can be removed by dialysis
■ Volume overload
■ Uremia (BUN > 100 mg/dL) or uremic symptoms (pericarditis, encephalopathy, bleeding, dyscrasia, nausea, vomiting, pruritus)
20-4. Chronic Kidney Disease
Definition and Classification of Chronic Kidney Disease
Chronic kidney disease (CKD) is defined as at least a 3-month period of kidney damage with or without a decrease in GFR or a GFR < 60 mL/min/1.73 m2 for greater than 3 months, with implications for health. Kidney damage is defined as pathologic abnormalities or markers of damage, including abnormalities in blood or urine tests or in imaging studies.
The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines classify CKD into five stages on the basis of kidney damage and GFR (Table 20-4). ESRD occurs when patients require renal replacement therapy (either dialysis or transplantation) to sustain life. Based on the more recent recommendations from KDIGO guidelines for evaluation and management of CKD, CKD is classified by cause of kidney disease, GFR category, and albuminuria level (Table 20-5). This classification is referred to as CGA staging (Cause, GFR, Albuminuria). This section will refer to the KDOQI stage (e.g., stage 4 CKD) and corresponding KDIGO category (e.g., G4).
Table 20-4. KDOQI Stages of CKD
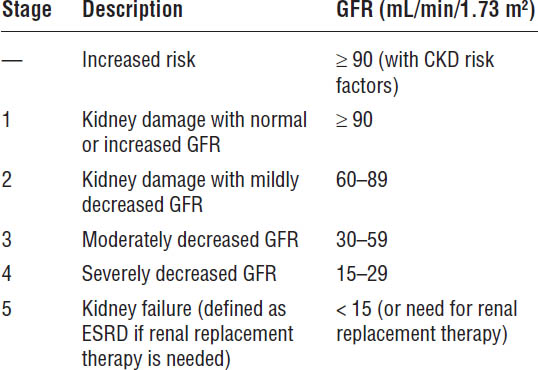
Adapted from National Kidney Foundation, 2002.
Epidemiology of CKD
Incidence and prevalence
Approximately 26 million American adults have CKD. The number of patients with CKD continues to increase, with a 50% increase in the number of patients with ESRD expected by 2020. The incidence of CKD is approximately four times higher in the African American population. The incidence is greatest in individuals age 45–64.
Approximately 600,000 patients are being treated for ESRD (including patients receiving hemodialysis, peritoneal dialysis, and transplantation).
Mortality
Life expectancy is four to five times shorter in dialysis patients than in the general population. The primary causes of death in the ESRD population are cardiovascular diseases and infection. Comorbidities, estimated GFR, and albuminuria at initiation of dialysis are strong predictors of mortality in the dialysis population.
Clinical Presentation
■ Changes in urine output (may not occur in earlier stages of CKD)
■ Foaming of urine, which indicates proteinuria
• Table 20-5 shows levels of albuminuria. If total protein levels are measured (including albumin and other proteins), different thresholds apply. A protein excretion ratio (PER) < 150 mg/24 h and a protein-to-creatinine ratio (PCR) of < 150 mg/g are considered normal. A PER or PCR above 150 is considered moderately increased, whereas levels above 500 are considered severely increased.
Table 20-5. KDIGO Classification of CKD Based on GFR and Albuminuriaa
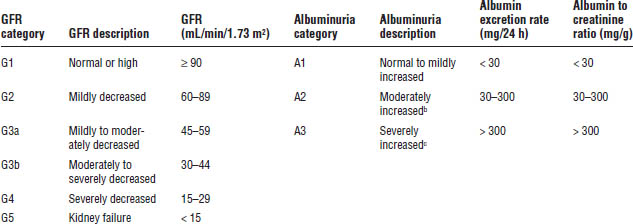
Adapted from KDIGO Chronic Kidney Disease Work Group, 2012.
a. GFR categories are similar to KDOQI stages with the exception that Stage 3 is divided into two subcategories by KDIGO (G3a and G3b).
b. Also referred to as microalbuminuria, although KDIGO does not advocate this terminology.
c. Also referred to as albuminuria (or overt proteinuria if proteins other than albumin are measured), although KDIGO does not advocate this terminology.
• Note: Nephrotic syndrome is a clinical syndrome associated with total protein in the urine in amounts > 3.5 g/day (referred to as nephrotic range proteinuria), hypoalbuminemia, edema, and hyperlipidemia.
■ Increased blood pressure (hypertension is a common etiology and result of CKD)
■ Signs and symptoms of hyperglycemia and glucosuria (diabetes is a common etiology)
■ Signs and symptoms associated with fluid and electrolyte abnormalities (e.g., hyperkalemia, fluid overload; see Chapter 21 on fluids and electrolytes)
■ Development of secondary complications of CKD:
• Anemia: Decreased hemoglobin and hematocrit, iron deficiency also common
• CKD Mineral and Bone Disorder: Increased serum phosphorus, decreased serum calcium (may shift to hypercalcemia as kidney disease progresses), increased intact parathyroid hormone (iPTH), vitamin D deficiency, increased fibroblast growth factor-23 (FGF-23)
• Metabolic acidosis: Decreased serum bicarbonate, increased anion gap
• Malnutrition: Decreased albumin and prealbumin (see Chapter 22 on nutrition)
■ Signs of uremia (see Section 20-1) in later stages of CKD, that is, stage 4 CKD (G4) and stage 5 CKD (G5)
Pathophysiology of Progressive Kidney Disease and Selected Secondary Complications
Progressive kidney disease
Progressive loss of nephron function results in adaptive changes in remaining nephrons to increase single nephron glomerular filtration pressure. Over time, the compensatory increase in single nephron GFR leads to hypertrophy from sustained increases in pressure and loss of individual nephron function.
Proteinuria, one of the initial diagnostic signs, may also contribute to the progressive decline in kidney function. Loss of kidney function is usually irreversible.
Etiology of progressive kidney disease
Each of the following may result in damage to the kidney that over time leads to a decrease in functioning nephrons and in total GFR:
■ Diabetes (accounts for primary cause in 44% of patients with ESRD)
■ Hypertension (accounts for primary cause in 26% of patients with ESRD)
■ Glomerulonephritis (multiple causes, e.g., systemic lupus erythematosus)
■ Polycystic kidney disease
■ HIV (human immunodeficiency virus) nephropathy
■ Other contributing factors (smoking, obesity, genetic factors, gender differences)
Anemia of CKD
The primary etiology is a decrease in production of the hormone erythropoietin by the kidney as kidney disease progresses. More than 90% of erythropoietin production occurs in the kidney and approximately 10% in the liver.
CKD results in a normochromic, normocytic anemia. Red blood cell lifespan is also decreased from 120 days to approximately 60 days in patients with kidney failure. Other contributors include iron deficiency and blood loss (e.g., from uremic bleeding, dialysis).
CKD Mineral and Bone Disorder
CKD mineral and bone disorder (CKD-MBD) includes abnormalities in parathyroid hormone (PTH), calcium, phosphorus, vitamin D, FGF-23, and bone turnover. Patients with CKD-MBD are also at risk for calcifications as kidney disease progresses.
As kidney function declines, phosphorus elimination decreases. Hyperphosphatemia causes a reciprocal decrease in serum calcium concentrations (hypocalcemia). Hypocalcemia stimulates the release of PTH by the parathyroid glands. Conversion of the vitamin D precursor (25-hydroxyvitamin D) to the active form (1,25-dihydroxyvitamin D3) occurs in the kidney. As kidney disease progresses, there is a decline in the 1α-hydroxylase enzyme that promotes the final hydroxylation step in the kidney, resulting in a deficiency in active vitamin D. Deficiencies in the precursor form of vitamin D have also been observed in CKD stage 3 (G3a and G3b) and stage 4 (G4). Active vitamin D (1,25-dihydroxyvitamin D3) promotes increased intestinal absorption of calcium and suppresses production of parathyroid hormone by the parathyroid gland; therefore, vitamin D deficiency leads to worsening secondary hyperparathyroidism.
Increased PTH promotes the following:
■ Decreased phosphorus reabsorption within the kidney
■ Increased calcium reabsorption by the kidney
■ Increased calcium mobilization from bone
■ Stimulated production of active vitamin D
As kidney disease progresses, the following occur:
■ Hyperphosphatemia and subsequent hypocalcemia progressively worsen, and secondary hyperparathyroidism becomes more severe.
■ The renal effects of PTH on phosphorus and calcium are no longer maintained, and PTH predominantly stimulates calcium resorption from bone.
■ Hyperphosphatemia also promotes an increase in FGF-23, produced and secreted primarily from osteocytes in bone, which reduces phosphorus by decreasing renal tubular reabsorption of phosphate and decreasing production of active vitamin D. Increases in FGF-23 are observed very early in the disease process.
■ Decreased production of active vitamin D worsens hypocalcemia and secondary hyperparathyroidism.
• In more severe CKD, stages 4 (G4) and 5 (G5), patients are prone to develop hypercalcemia, because of decreased renal elimination and use of calcium-containing phosphate binders.
• Patients with stage 5 CKD (G5) are at risk for calcifications and calciphylaxis.
■ Uncontrolled secondary hyperparathyroidism leads to hyperplasia of the parathyroid gland and renal osteodystrophy (a high bone turnover disease that results from sustained effects of PTH on bone).
Metabolic acidosis
■ Decreased excretion of acid by the kidney
■ Accumulation of endogenous acids attributable to impaired kidney function (e.g., phosphates, sulfates)
Diagnostic Criteria
Progressive kidney disease
There is a progressive increase in serum creatinine: > 1.1–1.2 mg/dL for females and > 1.2–1.3 mg/dL for males. Consider factors that may alter serum creatinine, such as decreased muscle mass and nutritional status.
There is a decreased GFR (see Tables 20-4 and 20-5 for CKD classifications). Consider the assessment method used to estimate creatinine clearance or GFR. The following methods are used to assess kidney function and may be used in patients with stable kidney function.
Creatinine clearance
■ Creatinine clearance (CrCl) is estimated using the Cockcroft–Gault equation (assumes stable kidney function):
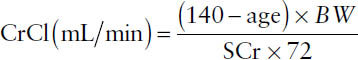
where BW = body weight in kg and SCr = serum creatinine. Adjusted body weight is recommended if a patient’s body weight is more than 30% above the ideal body weight. The result of the Cockcroft–Gault equation is multiplied by 0.85 for females.
■ CrCl is measured using urine collection methods:
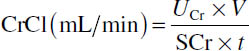
where
UCr = urinary creatinine concentration (mg/dL),
V = volume of urine (mL),
SCr = serum creatinine concentration (mg/dL), and
t = time period of urine collection (minutes).
Glomerular filtration rate
■ Modification of diet in renal disease (MDRD) abbreviated equation (use with nonstandardized serum creatinine):
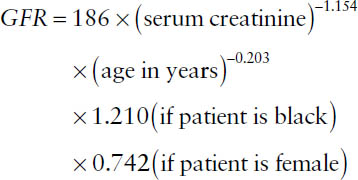
■ Reexpressed MDRD abbreviated equation (use with standardized serum creatinine):

Note: The MDRD equation is less accurate at GFRs above 60 mL/min/1.73 m2.
■ CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation (standardized serum creatinine should be used in this equation):
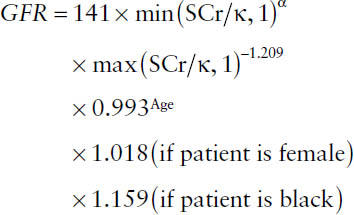
where κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates minimum of SCr/κ or 1, and max indicates maximum of SCr/κ or 1. Note: The CKD-EPI equation can be used at all ranges of GFR. In the pediatric population, use the Schwartz equation or the Counahan–Barratt equation.
Other diagnostic criteria for CKD include the following:
■ Proteinuria as assessed by the extent of albuminuria (Table 20-5) or proteinuria
■ Abnormal serum chemistries:
• Increased SCr and BUN
• The following abnormalities may indicate development of secondary complications of CKD: Increased potassium, decreased serum bicarbonate, increased phosphorus, decreased calcium (may have hypercalcemia in later stages of CKD)
Anemia of CKD
Testing for anemia is recommended in all patients with CKD. Guidelines for anemia management in patients with CKD recommend further evaluation for anemia when hemoglobin is < 12 g/dL in females and < 13.5 g/dL in males.
For iron deficiency, evaluate red blood cell indices and iron indices to identify iron deficiency as a contributing factor. Iron deficiency manifests as a microcytic anemia:
■ Red blood cell count: < 4.2 × 106 cells per mm2
■ Mean corpuscular volume: < 80 femtoliters
■ Serum iron: < 50 mg/dL
■ Total iron binding capacity: < 250 mg/dL
■ Transferrin saturation (TSat): < 16%
■ Serum ferritin: < 12 ng/mL
Transferrin saturation and serum ferritin should be maintained at higher values for CKD patients receiving erythropoiesis stimulating agents (TSat > 20% and serum ferritin > 100 ng/mL for CKD patients not on dialysis and for peritoneal dialysis patients, TSat > 20% and serum ferritin > 200 ng/mL for hemodialysis patients).
Evaluate for folate and vitamin B12 deficiencies (manifests as a macrocytic anemia), sources of blood loss (e.g., GI bleeding), and confounding disease states (e.g., cancer and HIV).
CKD-MBD
■ Serum phosphorus above normal range
■ Calcium abnormalities:
• Hypocalcemia: Corrected serum calcium < 8.5 mg/dL
• Hypercalcemia: Corrected calcium above the normal range (a concern in CKD stage 4 [G4] and stage 5 [G5]). Note: Corrected calcium = measured serum calcium + 0.8 × (normal serum albumin − measured serum albumin); normal serum albumin = 4 g/dL
■ Elevated calcium × phosphorus product: > 55 mg2/dL2 (elevated product increases risk for metastatic calcifications)
■ Elevated PTH: normal PTH ~ 10–60 pg/mL
■ Radiographic evidence of bone abnormalities (e.g., osteitis fibrosa cystica)
Metabolic acidosis
Serum bicarbonate (HCO3–) < 20 mEq/L.
Typically, the anion gap is increased: anion gap = [Na+] − ([Cl–] + [HCO3–]).
Signs and symptoms of chronic metabolic acidosis that develop as CKD progresses are generally not of the same magnitude as those of acute metabolic acidosis (e.g., hyperventilation, cardiovascular, and central nervous system manifestations).
Treatment Principles and Goals
Progressive kidney disease
Treatment principles
■ Control underlying cause of progressive CKD (e.g., hypertension and diabetes; see Chapters 12 and 17, respectively).
■ Meet blood pressure goals: < 140/90 mm Hg in patients with CKD if albumin excretion rate (AER) < 30 mg/24 h or < 130/80 mm Hg for patients with CKD and AER > 30 mg/24 h.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


